Research
The Cordes lab “Biophysical Chemistry” specializes in the development and application of novel spectroscopy and imaging techniques that allow to map structure and function of biomolecules and biochemical processes in space and time. For this the group uses a combination of optical techniques (single-molecule fluorescence spectroscopy & super-resolution imaging) with nanoscale sensors, i.e., fluorescent probes. In our current projects, we focus on molecular mechanisms of membrane transport and molecular motors (area 1), we are active in developing fluorescent probes and chemical biology approaches (area 2) and finally develop novel biophysical assays and instrumentation to characterize (bio)chemical processes and structures in vitro and in vivo (area 3).
Research Area 1: Molecular mechanisms of membrane transport
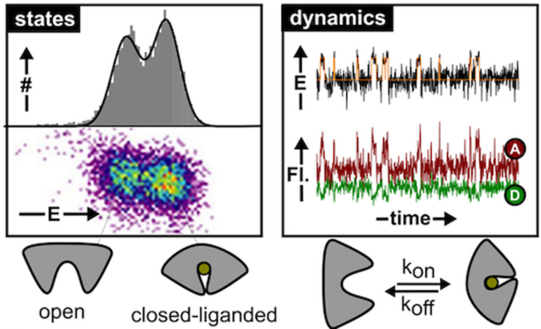
Membrane transport proteins play crucial roles in numerous cellular processes. Despite their importance, all proposed molecular models for transport are based on indirect evidence due to the inability of classical biophysical and biochemical techniques to directly visualize structural dynamics. This lack of data to validate mechanistic models for transport concerns both primary and secondary active transporters. The Cordes group is using single-molecule tools to decipher the molecular mechanisms of transport of these complex machines directly. This novel biophysical research area will support the development of new strategies against pathogenic bacteria or multi-drug resistant cancer cells.
Research Area 2: Probe development, spectroscopy & biolabelling
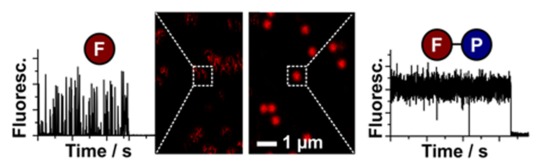
Fluorescence emission has evolved to an indispensable tool in the life sciences, e.g., as a general contrast mechanism for imaging, biochemical assays, medical screening, or DNA-sequencing. The merits of these applications and their information content are not limited by physical instruments, but by the performance of the employed fluorescent reporters. These intrinsically suffer from transient excursions to dark states limiting signal height and stability as well as from irreversible photochemical destruction (“photobleaching”) that restricts their observation time. The Cordes lab is developing novel fluorophores with self-healing or other functional properties imparted by the covalent linkage of e.g., photostabilizers to the fluorophore. These vastly improved properties have proven to be crucial for advanced fluorescence applications and super-resolution microscopy (STED & STORM). Current projects focus on optimization of chemical linkage of photostabilizer-dye conjugates and their application in live-cells.
Research Area 3: Development of biophysical assays and instrumentation
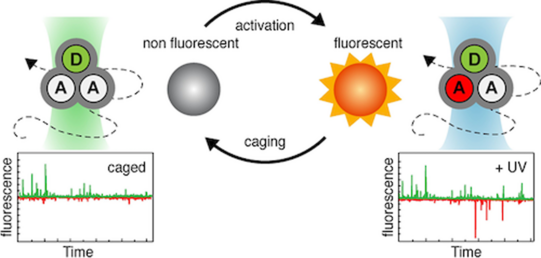
In relation to the mechanistic biochemical focus, the PBC group develops experimental tools based on (time-resolved) spectroscopy, photophysics and optical microscopy. One of the recent developments used a combination of photophysical (or photochemical) properties to obtain a multi-dimensional assay with higher information content: While a fluorescence resonance energy transfer process (FRET) reports on the distance (changes) between the two fluorophores the novel assay simultaneously allows to detect the presence of and distance to another yet unlabelled biomolecule via protein-induced fluorescence enhancement. Alternatively, we recently explored the use of caged fluorophores for smFRET experiments. Furthermore, the group is active in developing novel microscopy setups.