Research
The ubiquitin system
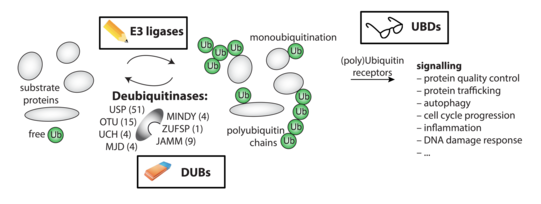
Post-translational modifications (PTMs) play a central role in coordinating intracellular processes. The ubiquitin protein is a post-translational modifier that can be attached to lysine side chains of substrate proteins by a cascade of E1/E2/E3 enzymes.
Signalling through ubiquitination is counteracted by specialised proteases termed de-ubiquitinating enzymes (DUBs), which selectively cleave the isopeptide bond between ubiquitin moieties in chains or between ubiquitin and substrate proteins.
More than 100,000 ubiquitinated sites have been mapped in the human proteome. Additional information can be encoded through differently linked ubiquitin chains and the interplay with other PTMs. This generates a complex and versatile intracellular signalling system with need for tight regulation of all enzymatic components.
Therapeutic opportunities in the ubiquitin system
Aberrant ubiquitin-dependent signaling has been implicated in the development of cancer, neurodegenerative and inflammatory diseases. Recent efforts to modulate DUB activity with small molecules have shown this to be a powerful strategy to amplify ubiquitin-dependent signalling and thereby alter key cellular processes, but compounds with high specificity and activity are lacking in many cases.
Moreover, for most pathologies it is unclear which DUB could be targeted for therapeutic purposes as for many insights into their functional roles and endogenous substrate recognition mechanisms are scarce.
Our mission
Our lab aims to understand mechanisms by which proteolytic enzymes in the Ubiquitin system operate, and to exploit these insights towards novel therapeutic approaches.
We believe that one promising way to shed light on the underlying complexity of the Ubiquitin system is to study its proteolytic components on the molecular and structural level.
We are guided by the vision that small molecules, which specifically target components of the ubiquitin system, can complement these efforts and provide a powerful means to indirectly modulate the levels of disease-relevant proteins in cells that are difficult to target directly, such as oncogenic transcription factors or frequently mutated enzymes.
Ultimately, we aim to molecularly connect these enzymes to pathologically relevant pathways and introduce well-characterised small molecule tools to interrogate their protein function in biological systems.
To achieve these goals, we work as a multidisciplinary team, integrating chemical biology, organic chemistry, protein biochemistry, structural biology and cellular assays.








