2024
62. T. Koike, J.-K. Yu, M. M. Hansmann*
Ph3PCN2: A stable reagent for carbon-atom transfer
Science 2024, 385, 305–311.
DOI: 10.1126/science.ado4564
Highlighted in the press release of TU Dortmund
Highlighted in Chemical & Engineering (C@EN) News
Highlighted in ChemistryWorld
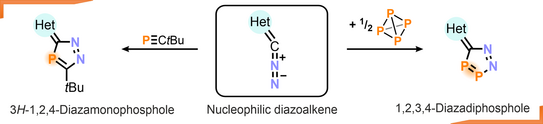
61. S. Hauer, J. Reitz, T. Koike, M. M. Hansmann*, R. Wolf*
Cycloadditions of Diazoalkenes with P4 and tBuCP: Access to Diazaphospholes
Angew. Chem. Int. Ed. 2024, e202410107, just accepted.
DOI: 10.1002/anie.202410107
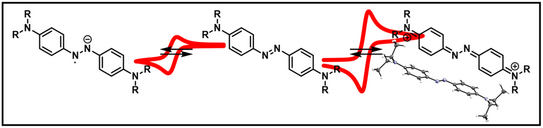
60. D. Schatz, M. E. Baumert, M. C. Kersten, F. M. Schneider, M. B. Nielsen, M. M. Hansmann, H. A. Wegner*
para-Aminoazobenzenes – Bipolar Redox-Active Molecules
Angew. Chem. Int. Ed. 2024, e202405618, just accepted.
DOI: 10.1002/anie.202405618
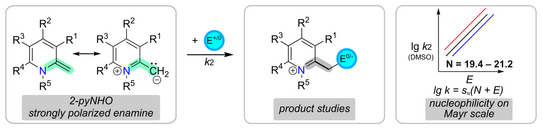
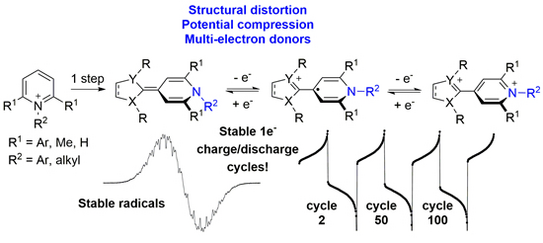
59. A. Behnke, A. Eitzinger, Y. He, P. W. Antoni, A. R. Ofial,* M. M. Hansmann*
2-Methylene-1,2-dihydropyridines (2-pyNHOs): Highly Nucleophilic Enamines
Eur. J. Org. Chem. 2024, asap.
DOI: 10.1002/ejoc.202400373
selected as VIP paper; Invited contribution to a special collection "physical organic chemistry"
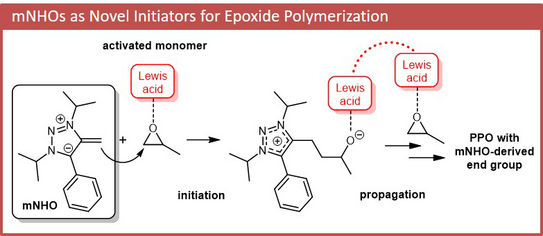
58. I. Haug, J. Reitz, C. Ziane, M. R. Buchmeister, M. M. Hansmann*, S. Naumann*
Mesoionic N-Heterocyclic Olefins as Initiators for the Lewis Pair Polymerization of Epoxides
Macromol. Rapid Commun. 2024, Volume (45), 2300716 (early view).
DOI: doi/10.1002/marc.202300716
"Part of the hot topic: Organocatalysis" (Link: aces.onlinelibrary.wiley.com/doi/toc/10.1002/(ISSN)1861-471X.hottopic-organocatalysis?page=2)
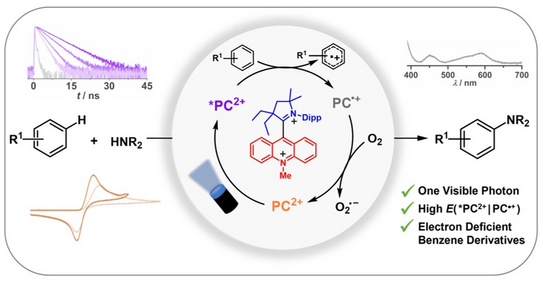
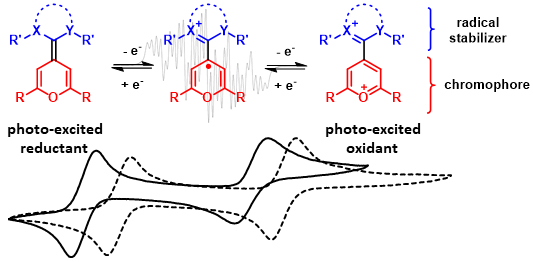
57. S. C. Sau, M. Schmitz, C. Burdenski, M. Baumert, P. W. Antoni, C. Kerzig,* M. M. Hansmann*
Dicationic Acridinium/Carbene Hybrids as Strongly Oxidizing Photocatalysts
J. Am. Chem. Soc. 2024, 146, 3416–3426
DOI: 10.1021/jacs.3c12766
(preprint: ChemRxiv DOI: 10.26434/chemrxiv-2023-qjwlc)
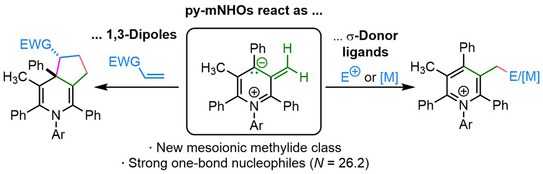
56. Q. Sun, A. Eitzinger, R. Esken, P. W. Antoni, R. J. Mayer,* A. R. Ofial,* M. M. Hansmann*
Pyridinium-Derived Mesoionic N-Heterocyclic Olefins (py-mNHOs)
Angew. Chem. Int. Ed. 2024, 63, e202318283.
DOI: 10.1002/anie.202318283
Angew. Chem. 2024, 136, e202318283.
DOI: 10.1002/ange.202318283
2023
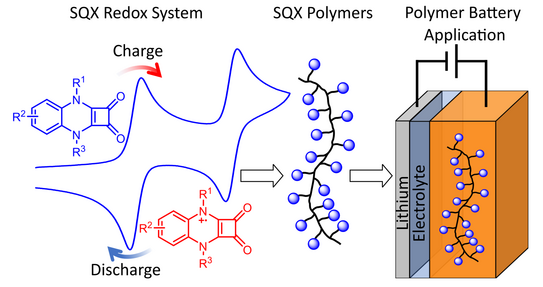
55. M. Baumert, V. Le, P.-H. Su, Y. Akae, D. Bresser*, P. Théato*, M. M. Hansmann*
From Squaric Acid Amides (SQAs) to Quinoxaline-Based SQAs-Evolution of a Redox-Active Cathode Material for Organic Polymer Batteries
J. Am. Chem. Soc. 2023, 145, 23334–23345.
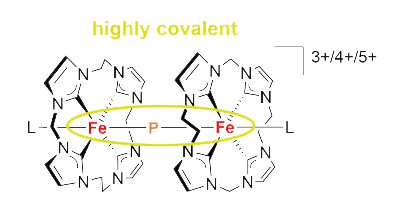
DOI: 10.1021/jacs.3c09153
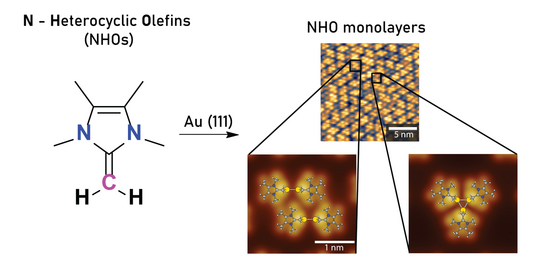
54. I. Berg, L. Schio, J. Reitz, E. Molteni, L. Lahav, C. G. Bolaños, A. Goldoni, C. Grazioli, G. Fratesi, M. M. Hansmann, L. Floreano, E. Gross*
Self-Assembled Monolayers of N-Heterocyclic Olefins on Au (111)
Angew. Chem. Int. Ed. 2023, 62, e202311832. (hot paper)
DOI: 10.1002/anie.202311832
Angew. Chem. 2023, 135, e202311832. (hot paper)
DOI: 10.1002/ange.202311832
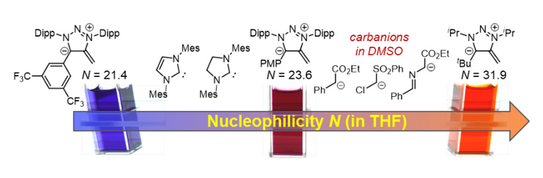
53. A. Eitzinger, J. Reitz, P. W. Antoni, H. Mayr, A. R. Ofial,* Max M. Hansmann*
Pushing the Upper Limit of Nucleophilicity Scales by Mesoionic N-Heterocyclic Olefins
Angew. Chem. Int. Ed. 2023, 62, e202309790 (hot paper)
DOI: 10.1002/anie.202309790
Angew. Chem. 2023, 135, e202309790 (hot paper)
DOI: 10.1002/ange.202309790
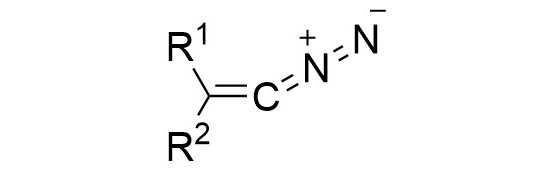
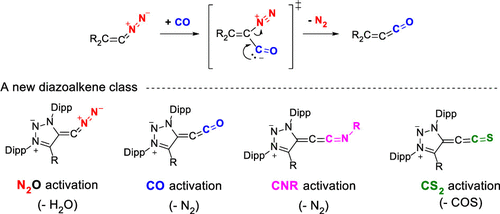
52. M. M. Hansmann*
Diazoalkenes – From an Elusive Intermediate to a Stable Substance Class in Organic Chemistry
Angew. Chem. Int. Ed. 2023, 62, e202304574
DOI: 10.1002/anie.202304574
Angew. Chem. 2023, 135, e202304574
DOI: 10.1002/ange.202304574
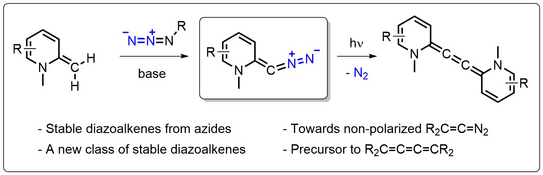
51. J. Reitz, P. W. Antoni, J. J. Holstein, M. M. Hansmann*
Room-Temperature-Stable Diazoalkenes by Diazo Transfer from Azides: Pyridine-Derived Diazoalkenes
Angew. Chem. Int. Ed. 2023, 62, e202301486
DOI: 10.1002/anie.202301486
Angew. Chem. 2023, 135, e202301486
DOI: 10.1002/ange.202301486
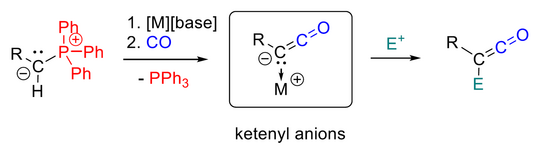
50. M. M. Hansmann*
Transition Metal-Free Activation of Carbon Monoxide: Ketenyl Anions by PPh3/CO Exchange (Highlight)
Angew. Chem. Int. Ed. 2023, 62, e202301826
DOI: 10.1002/anie.202301826
Angew. Chem. 2023, 135, e202301826
DOI: 10.1002/ange.202301826
2022
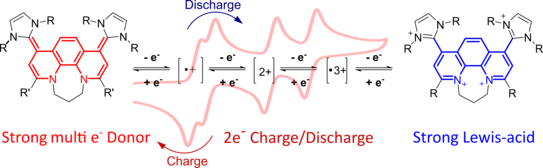
49. P. W. Antoni, C. Golz, M. M. Hansmann*
Organic Four-Electron Redox Systems Based on Bipyridine and Phenanthroline Carbene Architectures
Angew. Chem. Int. Ed. 2022, 61, e202203064.
DOI: 10.1002/anie.202203064
Angew. Chem. 2022, 134, e202203064
DOI: 10.1002/ange.202203064
2021
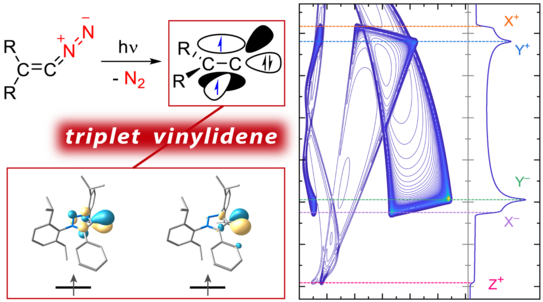
48. Y. Kutin, J. Reitz, P. W. Antoni, A. Savitsky, D. A. Pantazis*, M. Kasanmascheff*, M. M. Hansmann*
Characterization of a Triplet Vinylidene
J. Am. Chem. Soc. 2021, 143, 21410-21415.
DOI: 10.1021/jacs.1c11062
47. P. W. Antoni, J. Reitz, M. M. Hansmann*
N2/CO Exchange at a Vinylidene Carbon Center: Stable Alkylidene Ketenes and Alkylidene Thioketenes from 1,2,3-Triazole Derived Diazoalkenes
J. Am. Chem. Soc. 2021, 143, 12878–12885.
DOI: 10.1021/jacs.1c06906.
Highlighted in "JACS Spotlights" J. Am. Chem. Soc. 2021, 143, 12420–12421.
DOI: 10.1021/jacs.1c08248
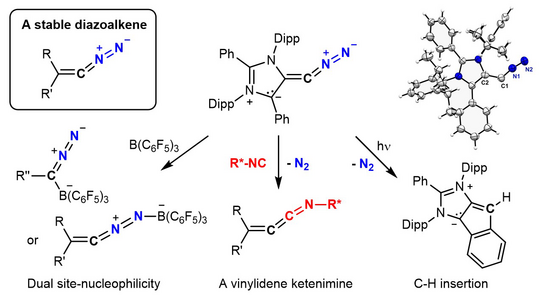
46. P. W. Antoni, C. Golz, J. J. Holstein, D. A. Pantazis, M. M. Hansmann*
Isolation and reactivity of an elusive diazoalkene
Nat. Chem. 2021, 13, 587–593.
DOI: 10.1038/s41557-021-00675-5.
Highlighted in “Notizen aus der Chemie” Nachr. Chem. 2021, 69 (Juli), 48.
Highlighted in „Trendberichte 2021: Anorganische Chemie“ M. Fischer, D. Heift, Nachr. Chem. 2022, 70 (Feb.), 40-51.
Highlighted in Nat. Chem. 2021, 13, 1030-1032 (DOI: 10.1038/s41557-021-00811-1)
Highlighted in press release of TU Dortmund
Highlighted in the press release of the Federal Ministry of Education and Research (BMBF)
2020
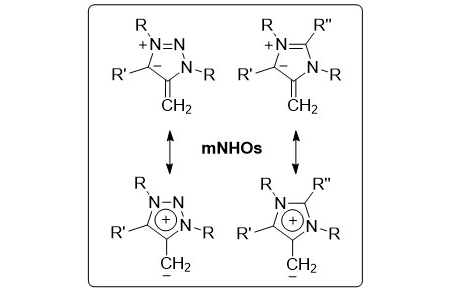
45. M. M. Hansmann*, P. W. Antoni, H. Pesch
Stable Mesoionic N‐Heterocyclic Olefins (mNHOs)
Angew. Chem. Int. Ed. 2020, 59, 5782-5787.
(DOI: 10.1002/anie.201914571)
Angew. Chem. 2020, 132, 5831-5836.
(DOI: 10.1002/ange.201914571)
Highlighted in „Trendberichte 2020: Anorganische Chemie“ C. Hering-Junghans, C. Sindlinger, Nachr. Chem. 2021, 69, 52-66.
44. J. Messelberger, A. Grünwald, S. J. Goodner, F. Zeilinger, P. Pinter, M. E. Miehlich, F. W. Heinemann, M. M. Hansmann, D. Munz*
Aromaticity and Sterics Control Whether a Cationic Olefin Radical is Resistant to Disproportionation
Chem. Sci. 2020, 11, 4138-4149.
(DOI: 10.1039/D0SC00699H)
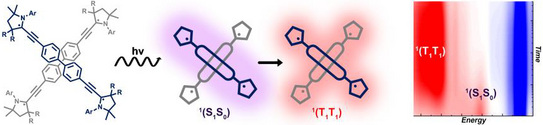
43. T. Ullrich, P. Pinter, J. Messelberger, P. Haines, R. Kaur, M. M. Hansmann*, D. Munz*, D.M. Guldi*
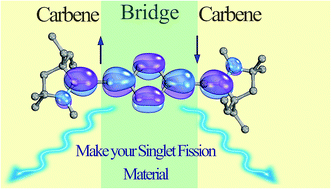
Singlet Fission in Carbene Derived Diradicaloids
Angew. Chem. Int. Ed. 2020, 59, 7906-7914.
(DOI: 10.1002/anie.202001286)
Angew. Chem. 2020, 132, 7980-7988.
(DOI: 10.1002/ange.202001286)
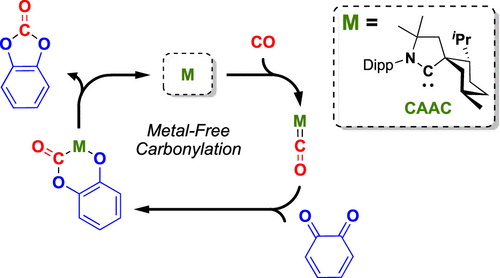
42. J. L. Peltier, E. Tomás-Mendivil, D. R. Tolentino, M. M. Hansmann, R. Jazzar, G. Bertrand*
Realizing Metal-Free Carbene-Catalyzed Carbonylation Reactions with CO
J. Am. Chem. Soc. 2020, 142, 18336-18340.
DOI: 10.1021/jacs.0c09938
2019
41. M. Gosh, H. H. Cramer, S. Dechert, S. Demeshko, M. John, M. M. Hansmann, S. Ye*, F. Meyer*
A μ-Phosphido Diiron Dumbbell in Multiple Oxidation States
Angew. Chem. Int. Ed. 2019, 58, 14349–14356.
(DOI: 10.1002/anie.201908213)
Angew. Chem. 2019, 131, 14487–14494.
(DOI: 10.1002/ange.201908213)
40. P. W. Antoni, T. Bruckhoff, M. M. Hansmann*
Organic Redox-Systems Based on Pyridinium-Carbene Hybrids
J. Am. Chem. Soc. 2019, 141, 9701–9711.
(DOI: 10.1021/jacs.9b04249)
2018
39. P. W. Antoni, M. M. Hansmann*
Pyrylenes: A New Class of Tunable, Redox-Switchable, Photoexcitable Pyrylium-Carbene Hybrids with Three Stable Redox-States
J. Am. Chem. Soc. 2018, 140, 14823–14835.
(DOI: 10.1021/jacs.8b08545)
38. J. Messelberger, A. Grünwald, P. Pinter, M. M. Hansmann, D. Munz*
Carbene derived diradicaloids – building blocks for singlet fission?
Chem. Sci. 2018, 9, 6107-6117.
(DOI: 10.1039/C8SC01999A)
37. M. M. Hansmann*
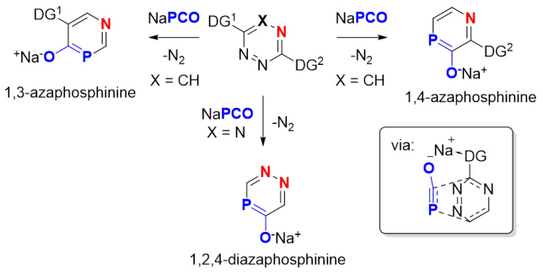
Synthesis of Azaphosphinines by Directed Inverse Electron Demand Hetero‐Diels‐Alder Reactions with Na(OCP)
Chem. Eur. J. 2018, 24, 11573-11577.
(DOI: 10.1002/chem.201802173)
36. A. Hinz, M. M. Hansmann, G. Bertrand*, J. M. Goicoechea*
Intercepting a Transient Phosphino-Arsinidene
Chem. Eur. J. 2018, 24, 9514-9519. (DOI: 10.1002/chem.201802175)
35. M. M. Hansmann, M. Melaimi, D. Munz, G. Bertrand*
A Modular Approach to Kekulé Diradicaloids Derived from Cyclic (Alkyl)(amino)carbenes
J. Am. Chem. Soc. 2018, 140, 2546-2554. (DOI: 10.1021/jacs.7b11183)
34. M. M. Hansmann, M. Melaimi, G. Bertrand*
Organic Mixed Valence Compounds Derived from Cyclic (Alkyl)(amino)carbenes
J. Am. Chem. Soc. 2018, 140, 2206-2213. (DOI: 10.1021/jacs.7b11184)
2017
33. M. M. Hansmann, M. Melaimi, G. Bertrand*
Crystalline Monomeric Allenyl/Propargyl Radical
J. Am. Chem. Soc. 2017, 139, 15620-15623. (DOI: 10.1021/jacs.7b09622)
32. E. Tomás-Mendivil, M. M. Hansmann, C. M. Weinstein, R. Jazzar, M. Melaimi, G. Bertrand*
Bicyclic (Alkyl)(amino)carbenes (BICAACs): Stable Carbenes more Ambiphilic than CAACs
J. Am. Chem. Soc. 2017, 139, 7753-7756. (DOI: 10.1021/jacs.7b04640)
31. M. M. Hansmann, D. A. Ruiz, L. Liu, R. Jazzar, G. Bertrand*
(Phosphanyl)phosphaketenes as Building Blocks for Novel Phosphorus Heterocycles
Chem. Sci. 2017, 8, 3720-3725. (DOI: 10.1039/C7SC00300E)
30. S. Arndt, M. M. Hansmann, M. Rudolph, F. Rominger, A. S. K. Hashmi*
Direct Access to π-Extended Phosphindolium Salts by Simple Proton-Induced Cyclization of (o-Alkynylphenyl)-phosphanes
Chem. Eur. J. 2017, 23, 5429-5433. DOI: 10.1002/chem.201700889)
29. S. Tšupova, M. M. Hansmann, M. Rudolph, F. Rominger, A. S. K. Hashmi*
Gold-Catalysed Formal Cyclisation/Dimerization of Thiophene-Tethered Diynes
Chem. Eur. J. 2017, 23, 5716-5721. (DOI: 10.1002/chem.201700061)
28. S. Arndt, M. M. Hansmann*, P. Motloch, M. Rudolph, F. Rominger, A. S. K. Hashmi*
Intramolecular anti-Phosphinoauration of Alkynes: An FLP-Motivated Approach to Stable Aurated Phosphindolium Complexes
Chem. Eur. J. 2017, 23, 2542-2547. (DOI: 10.1002/chem.201605914)
2016
27. M. M. Hansmann, G. Bertrand*
Transition-Metal-like Behavior of Main Group Elements: Ligand Exchange at a Phosphinidene
J. Am. Chem. Soc. 2016, 138, 15885-15888. (DOI: 10.1021/jacs.6b11496)
26. G. Kleinhans, M. M. Hansmann, G. Guisado-Barrios, D. C. Liles, G. Bertrand, D. I. Bezuidenhout*
Nucleophilic T-shaped (LXL)Au(I)-pincer complexes: Protonation and Alkylation
J. Am. Chem. Soc. 2016, 138, 15873-15876. (DOI: 10.1021/jacs.6b11359)
Highlighted in ACS Spotlight: J. Am. Chem. Soc. 2016, 138, 16567–16567.
25. S. Tšupova, M. M. Hansmann, M. Rudolph, F. Rominger, A. S. K. Hashmi*
New pathways for the Dual Gold Catalyzed Cyclization of Diynes
Chem. Eur. J. 2016, 22, 16286-16291. (DOI: 10.1002/chem.201602873)
24. L. C. Wilkins, J. R. Lawson, P. Wieneke, F. Rominger, A. S. K. Hashmi, M. M. Hansmann, R. L. Melen*
The Propargyl Rearrangement to Functionalised Allyl- Boron and Borocation Compounds
Chem. Eur. J. 2016, 22, 14618–14624. (DOI: 10.1002/chem.201602719)
23. M. M. Hansmann, R. Jazzar, G. Bertrand*
Singlet (Phosphino)phosphinidenes are Electrophilic
J. Am. Chem. Soc. 2016, 138, 8356-8359. (DOI: 10.1021/jacs.6b04232)
22. M. M. Hansmann, A. López-Andarias, E. Rettenmeier, C. Egler-Lucas, F. Rominger, A. S. K. Hashmi,* C. Romero-Nieto*
B(C6F5)3: A Lewis acid that brings the light to the solid state
Angew. Chem. Int. Ed. 2016, 55, 1196-1199. (DOI: 10.1002/anie.201508461)
Erzeugung von Festkörperlumineszenz durch Koordination der Lewis-Säure B(C6F5)3 an nicht-emittierende Aldehyde
Angew. Chem. 2016, 128, 1212-1216. (DOI: 10.1002/ange.201508461)
(selected as hot paper); Highlighted in “Cutting-Edge Chemistry“ by the ACS.
21. L. C. Wilkins, H. B. Hamilton, B. M. Kariuki, A. S. K. Hashmi, M. M. Hansmann, R. L. Melen*
Lewis acid-base 1,2-addition reactions: Synthesis of pyrylium borates from en-ynoate precursors.
Dalton Trans. 2016, 45, 5929-5932. (DOI: 10.1039/C5DT03340C
2015
20. M. M. Hansmann*, R. L. Melen, M. Rudolph, F. Rominger, H. Wadepohl, D. W. Stephan*, A. S. K. Hashmi*
Cyclopropanation / Carboboration Reactions of Enynes with B(C6F5)3
J. Am. Chem. Soc. 2015, 137, 15469-15477. (DOI: 10.1021/jacs.5b09311)
19. L. C. Wilkins, P. Wieneke, P. D. Newman, B. M. Kariuki, F. Rominger, A. S. K. Hashmi, M. M. Hansmann*, R. L. Melen*
Pathways to functionalized heterocycles: The propargyl rearrangement using B(C6F5)3
Organometallics 2015, 34, 5298-5309. (DOI: 10.1021/acs.organomet.5b00753)
18. E. Rettenmeier, M. M. Hansmann, A. Ahrens, K. Rübenacker, T. Saboo, J. Massholder, C. Meier, M. Rudolph, F. Rominger, A. S. K. Hashmi*
Insights into the Gold-Catalyzed Propargyl Ester Rearrangement/ Tandem Cyclization Sequence: Radical versus Gold Catalysis — Myers–Saito- versus Schmittel-Type Cyclization
Chem. Eur. J. 2015, 21, 14401-14409. (DOI: 10.1002/chem.201501725)
17. R. L. Melen,* L. C. Wilkins, B. M. Kariuki, H. Wadepohl, L. H. Gade, A. S. K. Hashmi, D. W. Stephan, M. M. Hansmann*
Diverging Pathways in the Activation of Allenes with Lewis Acids and Bases: Addition, 1,2-Carboboration, and Cyclization
Organometallics 2015, 34, 4127-4137. (DOI: 10.1021/acs.organomet.5b00546)
2014
16. M. M. Hansmann*, F. Rominger, M. P. Boone, D. W. Stephan*, A. S. K. Hashmi*
Reactivity of Organogold Compounds with B(C6F5)3 – A Gold Boron Transmetalation via σ-B/π-Au Species
Organometallics 2014, 33, 4461-4470. (DOI: 10.1021/om5006885)
15. M. M. Hansmann, R. L. Melen, F. Rominger, A. S. K. Hashmi*, D. W. Stephan*
B(C6F5)3 Promoted Cyclisation of Internal Propargyl Esters: Structural Characterisation of 1,3-Dioxolium Compounds
Chem. Commun. 2014, 50, 7243-7245. (DOI: 10.1039/C4CC01370K) (selected as hot article)
14. T. Wang, S. Shi, M. M. Hansmann, E. Rettenmeier, M. Rudolph, A. S. K. Hashmi*
Synthesis of Highly Substituted 3-Formyl Furans via Gold(I)-Catalyzed Oxidation / 1,2-Alkynyl Migration / Cyclization Cascade
Angew. Chem. Int. Ed. 2014, 53, 3715-3719. (DOI: 10.1002/anie.201310146)
Synthese hochsubstituierter 3-Formylfurane über eine Kaskade aus Gold(I)-katalysierter Oxidation, 1,2-Alkinylwanderung und Cyclisierung
Angew. Chem. 2014, 126, 3789-3793. (DOI: 10.1002/ange.201310146)
Classified as highly cited paper by Elsevier
13. M. M. Hansmann, R. L. Melen, F. Rominger, A. S. K. Hashmi*, D. W. Stephan*
Activation of Alkynes with B(C6F5)3 - Boron Allylation Reagents Derived from Propargyl Esters
J. Am. Chem. Soc. 2014, 136, 777-782. (DOI: 10.1021/ja4110842)
12. M. M. Hansmann*, S. Tsupova, M. Rudolph, F. Rominger, A. S. K. Hashmi*
Gold-catalysed Cyclisation of Diynes: Controlling the Mode of 5-endo vs. 6-endo Cyclisation – An Experimental and Theoretical Case Study Utilizing Diethynylthiophenes
Chem. Eur. J. 2014, 20, 2215-2223. (DOI: 10.1002/chem.201302967)
2013
11. M. M. Hansmann, M. Pernpointner*, R. Döpp, A. S. K. Hashmi*
A Theoretical DFT-based and an Experimental Study of the Transmetalation Step in Au/Pd Mediated Cross-Coupling Reactions
Chem. Eur. J. 2013, 19, 15290-15303. (DOI: 10.1002/chem.201301840)
10. W. Yang, Y. Yu, T. Zhang, M. M. Hansmann, D. Pflästerer, A. S. K. Hashmi*
Gold-Catalyzed Highly Diastereoselective Synthesis of Functionalized 3,4-Disubstituted Butyrolactams via Phosphatyloxy or Carbonate Double Migrations
Adv. Synth. Catal. 2013, 335, 2037-2043. (DOI: 10.1002/adsc.201300158)
Highlighted in SynFacts: Synfacts 2013, 9, 1086.
9. R. L. Melen†, M. M. Hansmann†, A. J. Lough, A. S. K. Hashmi, D. W. Stephan*
Cyclisation versus 1,1-Carboboration: Reactions of B(C6F5)3 with Propargylamides
Chem. Eur. J. 2013, 19, 11928-11938. (DOI: 10.1002/chem.201301899) († equal contribution)
8. M. M. Hansmann, A. S. K. Hashmi*, M. Lautens*
Gold meets Rhodium: Tandem One-Pot Synthesis of β-Disubstituted Ketones via Meyer-Schuster Rearrangement and Asymmetric 1,4-Addition
Org. Lett. 2013, 15, 3226-3229. (DOI: 10.1021/ol4011739)
Highlighted in SynFacts: Synfacts 2013, 9, 1087.
Highlighted on www.organic-chemistry.org
7. M. M. Hansmann*, M. Rudolph, F. Rominger, A. S. K. Hashmi*
Mechanistic switch in dual gold catalysis of diynes: C(sp3)-H activation through bifurcation – vinylidene versus carbene pathways
Angew. Chem. Int. Ed. 2013, 52, 2593-2598. (DOI: 10.1002/anie.201208777)
Mechanistisches Umschalten bei der dualen Goldkatalyse von Diinen: C(sp3)-H-Aktivierung über Bifurkation – Vinyliden- versus Carbenreaktionswege
Angew. Chem. 2013, 125, 2653-2659. (DOI: 10.1002/ange.201208777)
Classified as highly cited paper by Elsevier (> 100 citations)
6. M. M. Hansmann*, F. Rominger, A. S. K. Hashmi*
Gold-Allenylidenes – An experimental and theoretical study
Chem. Sci. 2013, 4, 1552-1559. (DOI: 10.1039/C3SC22227F)
5. A. S. K. Hashmi*, W. Yang, Y. Yu, M. M. Hansmann, M. Rudolph, F. Rominger
Gold-catalyzed formal 1,6-acyloxy migration leading to 3,4-disubstituted pyrrolidin-2-ones
Angew. Chem. Int. Ed. 2013, 52, 1329-1332. (DOI: 10.1002/anie.201207287)
Goldkatalysierte formale 1,6-Acyloxywanderung unter Bildung von 3,4-disubstituierten Pyrrolidin-2-onen
Angew. Chem. 2013, 125, 1368-1371. (DOI: 10.1002/ange.201207287)
Highlighted in SynFacts: Synfacts 2013, 9, 414.
2012
4. B. M. Trost*, D. A. Thaisrivongs, M. M. Hansmann
Tandem Palladium(0)- and Palladium(II)-catalyzed allylic alkylation through complementary redox cycles
Angew. Chem. Int. Ed. 2012, 51, 11522-11526. (DOI: 10.1002/anie.201204251)
Angew. Chem. 2012, 124, 11690-11694. (DOI: 10.1002/ange.201204251)
3. B. M. Trost*, M. M. Hansmann, D. A. Thaisrivongs
Palladium-catalyzed alkylation of 1,4-dienes by C–H activation
Angew. Chem. Int. Ed. 2012, 51, 4950-4953. (DOI: 10.1002/anie.201200601)
Angew. Chem. 2012, 124, 5034-5037. (DOI: 10.1002/ange.201200601)
2011
2. A. S. K. Hashmi*, M. Pernpointner, M. M. Hansmann
Theoretical insights into the superior activity of gold catalysts and reactions of organogold intermediates with electrophiles
Faraday Discuss. 2011, 152, 179-184. (DOI: 10.1039/C1FD00029B)
1. M. M. Hansmann, R. L. Melen, D. S. Wright*
Group 13 BN dehydrocoupling reagents, similar to transition metal catalysts but with unique reactivity
Chem. Sci. 2011, 2, 1554-1559. (DOI: 10.1039/C1SC00154J)
Patents
1. Novel compounds exhibiting photopysical properties upon formation of Lewis acid-base adducts using non-chelating boranes, method for producing the same and devices including the same.
M. M. Hansmann, A. S. K. Hashmi, C. Romero-Nieto, A. López-Andarias, E. Rettenmeier, C. Egler-Lucas
Patent registration WO 2017/016653 A1; PCT/EP2016/001254.
Non-Peer Reviewed Articles
M. M. Hansmann*
Blickpunkt Nachwuchs: Redoxsysteme und neue stabile Verbindungsklassen
Nachrichten aus der Chemie, 2022, 70, 80-81
DOI: 10.1002/nadc.20224125418