Research
Optogenetics
Optical control of enzymatic activity. Light is an important signal for inducing and regulating chemical reactions because it is orthogonal to other mechanisms of functional control such as small-molecule binding or protein-protein interaction, and it is applicable with high spatial and temporal precision. In nature, light is absorbed by photosensory domains which transmit the incoming signal via structural (steric, dynamic, or electronic) changes to a tethered protein. Several classes of photosensory proteins exist which differ with respect to photochemisry, chromophor and thus, absorbed light energy. Light-oxygen voltage (LOV) domains are among the best studied photoreceptors that are linked to effector domains such as kinases, phosphodiesterases, or DNA-binding proteins.
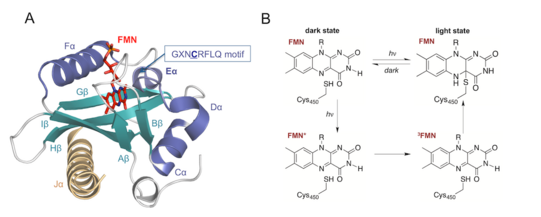
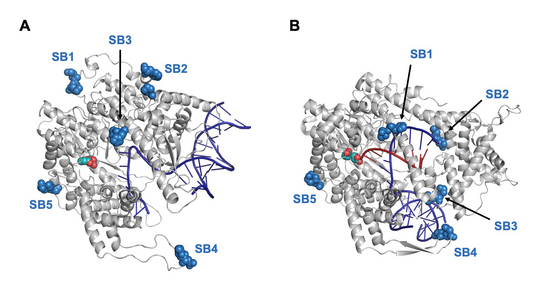
We are investigating how LOV domains can be combined with enzymes which are not naturally regulated by light. A recent example concerns nucleic acid polymerases, the core proteins that mediate replication of DNA and RNA in all types of cells. Our research aims at understanding mechanisms of allosteric protein switching even in proteins with complex catalytic activity and at generating tools for studying the roles of these enzymes in cellular, developmental and disease-related processes.
Biocatalysis
Enzymatic reaction cascades. Enzymes are very efficient catalysts and ideally suited for application in a sustainable chemistry because they typically accelerate reactions by factors of 108-1010, are applied at low mole percentage (10-3-10-4), are environmentally benign (completely biodegradable), act under mild conditions, and neither are restricted to their natural role nor to their natural substrate.
We are, for example, currently investigating whether the simple reaction
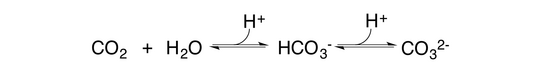
could be forced towards the production and utilization of carbonate using enzymatic catalysis. In the simplest case, carbonate could be precipitated but it may also be employed as a substrate in organic synthesis. Candidate enzymes for this reaction scheme include carbonic anhydrases as well as enzymes for the turnover of bicarbonate.