2026
76. M. Amann, M. Drosou, T. Al Said, A. Allgaier, Y. Kutin, P. W. Antoni, J. J. Holstein, M. Kasanmascheff, J. van Slageren, A. Schnegg*, D. A. Pantazis*, M. M. Hansmann*
Triplet Metallovinylidenes of Palladium and Platinum Based on a Chelating P/Diazoalkene Ligand
Angew. Chem. Int. Ed. 2026, 65, e16032.
DOI: 10.1002/anie.202516032
Angew. Chem. 2026, 138, e16032.
DOI: 10.1002/ange.202516032
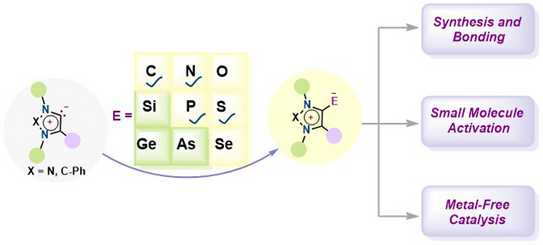
75. S. Maji, S. P, M. M. Hansmann*, S. K. Mandal*
Mesoionic N-Heterocyclic Olefins, Imines, Thiones, Phosphinidenes and Their Application in Catalysis
Angew. Chem. Int. Ed. 2026, 65, e20101.
DOI: 10.1002/anie.202520101
Angew. Chem. 2026, 138, e20101.
DOI: 10.1002/ange.202520101
2025
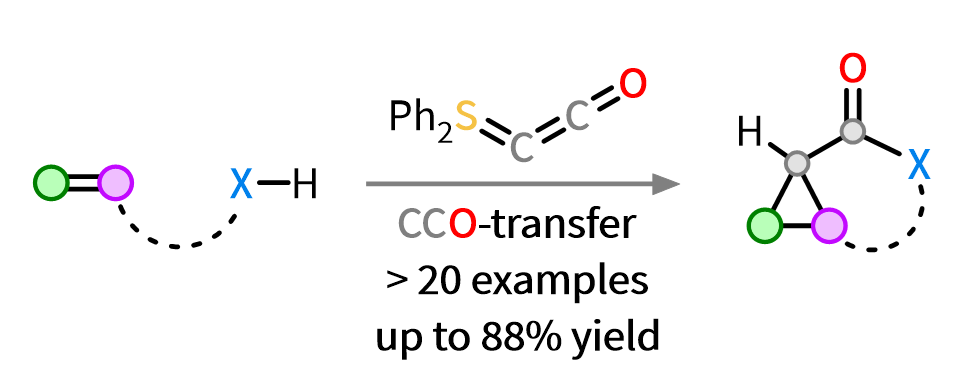
74. Q. Sun, J. Hauda, D. Tymann, P. W. Antoni, R. Goddard, M. M. Hansmann*
Ph2SCCO: A New Versatile CCO-Fragment Transfer Reagent
Angew. Chem. Int. Ed. 2025, 64, e202518689.
DOI: 10.1002/anie.202518689
Angew. Chem. 2025, 137, e202518689.
DOI: 10.1002/ange.202518689
73. S. Hauer, G. Balázs, J. Reitz, M. M. Hansmann, R. Wolf*
Coordination Studies of a Triazole-Substituted 3H-1,2,4-Diazaphosphole: C─C and C─N Bond Activation Induced by Cobalt(0) and Nickel(0) Complexes
Z. Anorg. Allg. Chem. 2025, e202500167, just accepted.
DOI: 10.1002/zaac.202500167
Special issue dedicated to Prof. Christian Limberg and Prof. Franc Meyer on the occasion of their 60th birthdays.
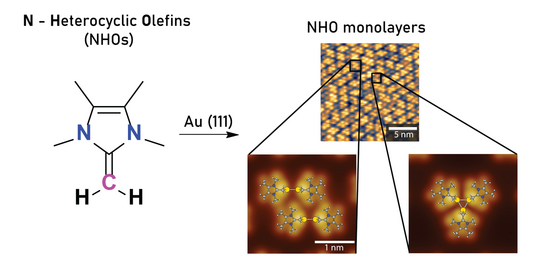
72. I. Berg, L. Schio, M. Alihosseini, J. Reitz, E. Molteni, S. Ma, C. G. Bolaños, A. Goldoni, C. Grazioli, M. M. Hansmann, G. Fratesi,* L. Floreano,* E. Gross*
Self-assembled monolayers of mesoionic triazolylidene dimers on Au(111)
Nanoscale 2025, 17, 25213–25226.
DOI: doi.org/10.1039/D5NR02802G
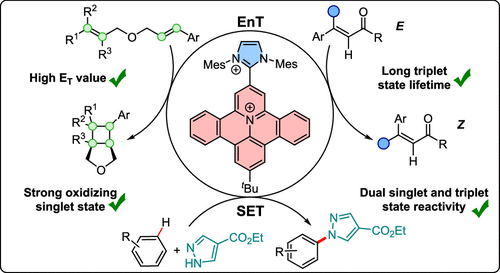
71. Ritu, M. Schmitz, C. Burdenski, P. W. Antoni, J. J. Holstein, C. Kerzig*, M. M. Hansmann*
π-Extended Heterocycle/Carbene Hybrids as Geometrically Constrained Dyes for TADF Energy and Electron Transfer Photocatalysis
ACS Catal. 2025, 15, 16718–16730
DOI: 10.1021/acscatal.5c06069
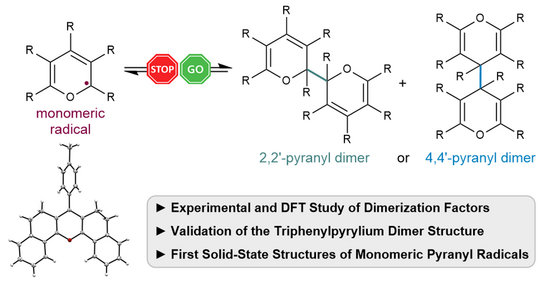
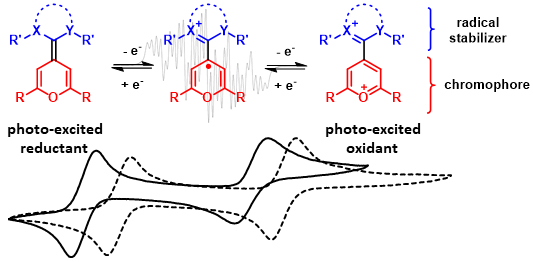
70. P. W. Antoni, A. Behnke, C. Golz, M. M. Hansmann*
Stabilization of Monomeric Pyranyl Radicals
J. Org. Chem. 2025, 90, 9108–9117.
DOI: 10.1021/acs.joc.5c00880
Invited contribution to a special collection "Physical Organic Chemistry: Never out of Style"
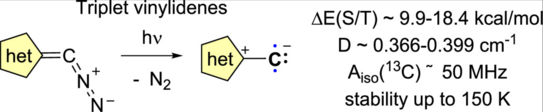
69. Y. Kutin, J. Reitz, M. Drosou, P. W. Antoni, Y. He, V. R. Selve, S. Boschmann, A. Savitsky, D. A. Pantazis*, M. Kasanmascheff*, Max M. Hansmann*
Triplet Vinylidenes Based on (Benz)imidazole and 1,2,3-Triazole N-Heterocycles
JACS Au 2025, 5, 2884–2897 .
DOI: 10.1021/jacsau.5c00491
Highlighted in “Notizen aus der Chemie” Nachr. Chem. 2025, 73, 52-55.
68. R. Esken, P. W. Antoni, Y. Lorenz, C. Burdenski, J.-L. Kirchhoff, C. Strohmann and M. M. Hansmann*
Imidazo[1,5-a]pyridines – A Versatile Platform for Structurally Distinct N-Heterocyclic Olefins and π-Extended Heterocycles
Angew. Chem. Int. Ed. 2025, 64, e202506305.
DOI: 10.1002/anie.202506305
Angew. Chem. 2025, 137, e202506305.
DOI: 10.1002/ange.202506305
Original data: DOI: 10.17877/RESOLV-2025-M9I8RY5L
Selected as Hot paper
Highlighted by D. Zhao, T. Xiao Synfacts 2025, 21, 785
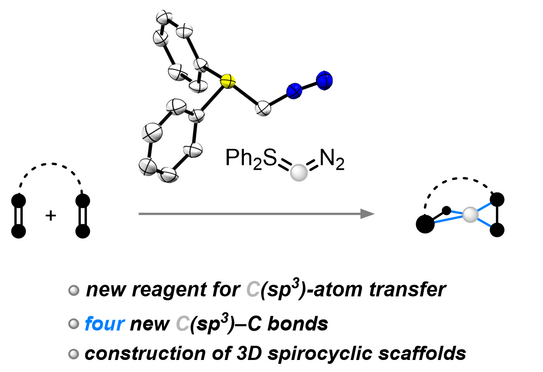
67. Q. Sun, J.-N. Belting, J. Hauda, D. Tymann, P. W. Antoni, R. Goddard, M. M. Hansmann*
Spiro-C(sp3)-atom transfer: Creating rigid three-dimensional structures with Ph2SCN2
Science 2025, 387, 885–892.
DOI: 10.1126/science.ads5974
Open access link
Highlighted in the press release of TU Dortmund
Highlighted in Chemical & Engineering (C@EN) News
Highlighted in Nat. Synth. 2025
Highlighted by D. Zhao, H. Li Synfacts 2025, 21, 677
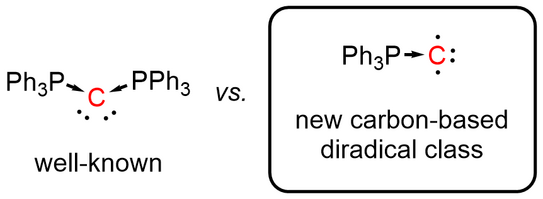
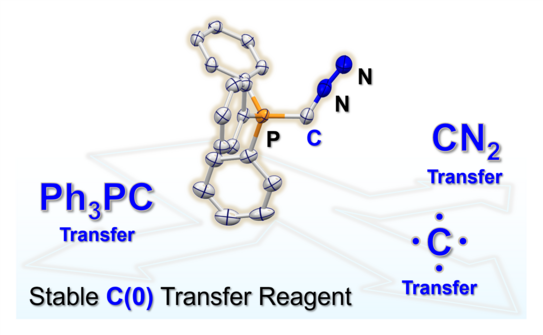
66. Y. Kutin, T. Koike, M. Drosou, A. Schnegg, D. Pantazis*, M. Kasanmascheff*, M. M. Hansmann*
Ph3PC – A Monosubstituted C(0) Atom in Its Triplet State
Angew. Chem. Int. Ed. 2025, 64, e202424166.
DOI: 10.1002/anie.202424166
Angew. Chem. 2025, 137, e202424166.
DOI: 10.1002/ange.202424166
Highlighted in Chemistryviews
Selected as VIP paper
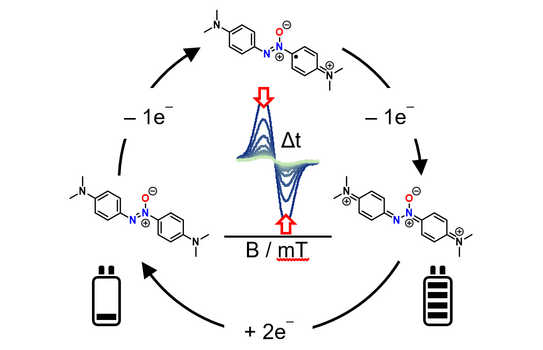
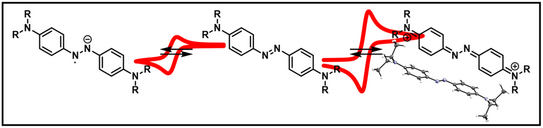
65. D. Schatz, C. Burdenski, F. M. Schneider, M. M. Hansmann, H. A. Wegner*
Amino-substituted Azoxybenzenes as Potential Redox-Active Catholyte Materials
Chem. Eur. J. 2025, e202404001, accepted article. DOI: 10.1002/chem.202404001
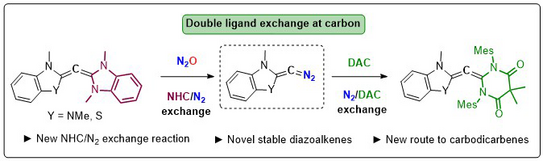
64. Y. He, Y. Lyu, D. Tymann, P. W. Antoni, M. M. Hansmann*
Cleavage of Carbodicarbenes with N2O for Accessing Stable Diazoalkenes: Two-fold ligand exchange at a C(0)-atom
Angew. Chem. Int. Ed. 2025, 64, e202415228.
DOI: 10.1002/anie.202415228
Angew. Chem. Int. Ed. 2025, 137, e202415228.
DOI:10.1002/ange.202415228
Selected as VIP paper
Featured as front cover
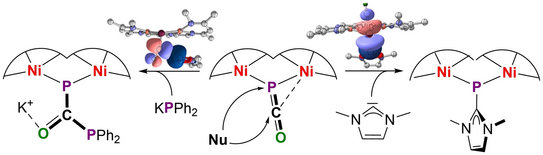
63. R. A. Schulz, U. S. Karaca, M. Diefenbach, N. J. A. Werthmann, S. Dechert, M. M. Hansmann, M. C. Holthausen*, F. Meyer*
From a P-Bridging Phosphaketene to μ-Phosphinidenide and μ-Diphosphaurea Units at a Dinickel Core
Chem. Eur. J. 2025, 31, e202404095.
DOI: 10.1002/chem.202404095
2024
62. T. Koike, J.-K. Yu, M. M. Hansmann*
Ph3PCN2: A stable reagent for carbon-atom transfer
Science 2024, 385, 305–311.
DOI: 10.1126/science.ado4564
Open access Link
Highlighted in the press release of TU Dortmund
Highlighted in Chemical & Engineering (C@EN) News
Highlighted in ChemistryWorld
Highlighted in Nachrichten aus der Chemie, 2024, 72, 50-53
Highlighted in: P. Richardson, Synfacts 2025, 21, 125
Highlighted in organic-chemistry.org
T. Koike, M. M. Hansmann
Taming the Carbon Atom - Reagent Development for Precise Molecular Editing. Explanatory Article, 2025 January Issue, Kagaku, Kagakudojin.
www.kagakudojin.co.jp/book/b656224.html
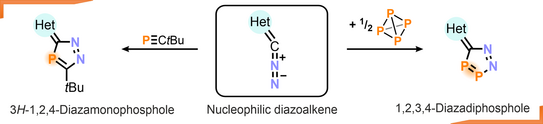
61. S. Hauer, J. Reitz, T. Koike, M. M. Hansmann*, R. Wolf*
Cycloadditions of Diazoalkenes with P4 and tBuCP: Access to Diazaphospholes
Angew. Chem. Int. Ed. 2024, 63, e202410107.
DOI: 10.1002/anie.202410107
Angew. Chem. 2024, 136, e202410107.
DOI: 10.1002/ange.202410107
60. D. Schatz, M. E. Baumert, M. C. Kersten, F. M. Schneider, M. B. Nielsen, M. M. Hansmann, H. A. Wegner*
para-Aminoazobenzenes – Bipolar Redox-Active Molecules
Angew. Chem. Int. Ed. 2024, 63, e202405618.
DOI: 10.1002/anie.202405618
Angew. Chem. 2024, 136, e202405618
DOI: 10.1002/ange.202405618
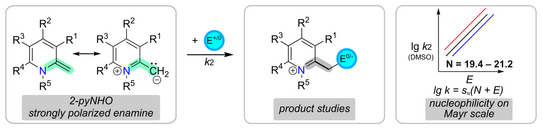
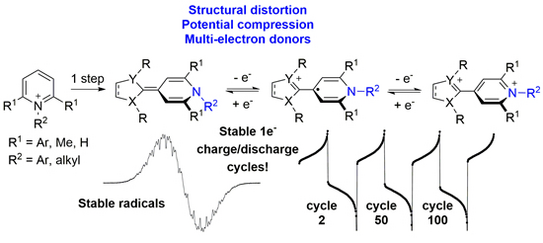
59. A. Behnke, A. Eitzinger, Y. He, P. W. Antoni, A. R. Ofial,* M. M. Hansmann*
2-Methylene-1,2-dihydropyridines (2-pyNHOs): Highly Nucleophilic Enamines
Eur. J. Org. Chem. 2024, 27, e202400373.
DOI: 10.1002/ejoc.202400373
selected as VIP paper; Invited contribution to a special collection "physical organic chemistry"
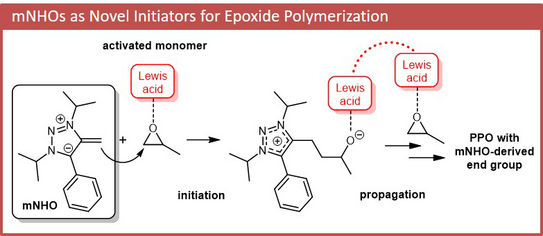
58. I. Haug, J. Reitz, C. Ziane, M. R. Buchmeister, M. M. Hansmann*, S. Naumann*
Mesoionic N-Heterocyclic Olefins as Initiators for the Lewis Pair Polymerization of Epoxides
Macromol. Rapid Commun. 2024, 45, 2300716.
DOI: doi/10.1002/marc.202300716
"Part of the hot topic: Organocatalysis"
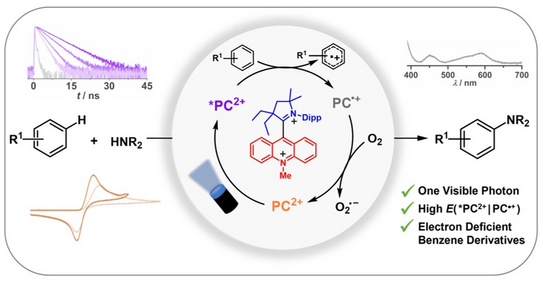
57. S. C. Sau, M. Schmitz, C. Burdenski, M. Baumert, P. W. Antoni, C. Kerzig,* M. M. Hansmann*
Dicationic Acridinium/Carbene Hybrids as Strongly Oxidizing Photocatalysts
J. Am. Chem. Soc. 2024, 146, 3416–3426
DOI: 10.1021/jacs.3c12766
(preprint: ChemRxiv DOI: 10.26434/chemrxiv-2023-qjwlc)
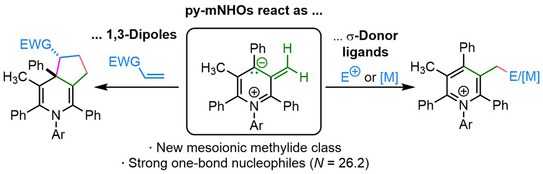
56. Q. Sun, A. Eitzinger, R. Esken, P. W. Antoni, R. J. Mayer,* A. R. Ofial,* M. M. Hansmann*
Pyridinium-Derived Mesoionic N-Heterocyclic Olefins (py-mNHOs)
Angew. Chem. Int. Ed. 2024, 63, e202318283.
DOI: 10.1002/anie.202318283
Angew. Chem. 2024, 136, e202318283.
DOI: 10.1002/ange.202318283
2023
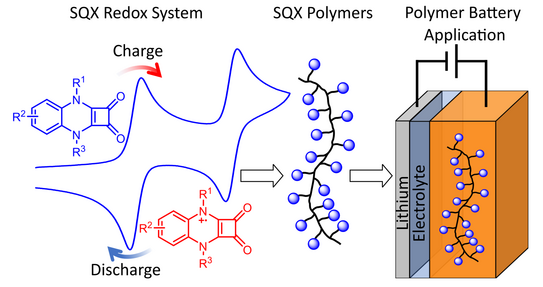
55. M. Baumert, V. Le, P.-H. Su, Y. Akae, D. Bresser*, P. Théato*, M. M. Hansmann*
From Squaric Acid Amides (SQAs) to Quinoxaline-Based SQAs-Evolution of a Redox-Active Cathode Material for Organic Polymer Batteries
J. Am. Chem. Soc. 2023, 145, 23334–23345.
DOI: 10.1021/jacs.3c09153
54. I. Berg, L. Schio, J. Reitz, E. Molteni, L. Lahav, C. G. Bolaños, A. Goldoni, C. Grazioli, G. Fratesi, M. M. Hansmann, L. Floreano, E. Gross*
Self-Assembled Monolayers of N-Heterocyclic Olefins on Au (111)
Angew. Chem. Int. Ed. 2023, 62, e202311832. (hot paper)
DOI: 10.1002/anie.202311832
Angew. Chem. 2023, 135, e202311832. (hot paper)
DOI: 10.1002/ange.202311832
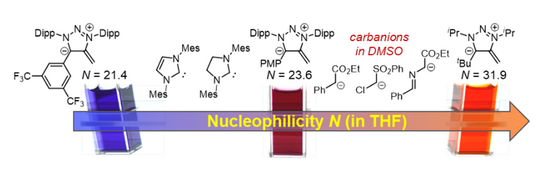
53. A. Eitzinger, J. Reitz, P. W. Antoni, H. Mayr, A. R. Ofial,* Max M. Hansmann*
Pushing the Upper Limit of Nucleophilicity Scales by Mesoionic N-Heterocyclic Olefins
Angew. Chem. Int. Ed. 2023, 62, e202309790 (hot paper)
DOI: 10.1002/anie.202309790
Angew. Chem. 2023, 135, e202309790 (hot paper)
DOI: 10.1002/ange.202309790
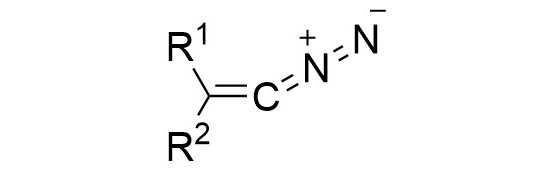
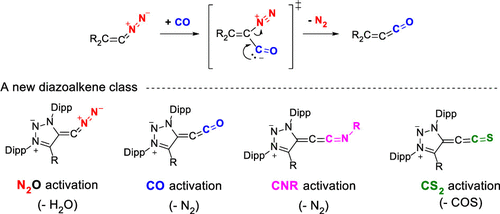
52. M. M. Hansmann*
Diazoalkenes – From an Elusive Intermediate to a Stable Substance Class in Organic Chemistry
Angew. Chem. Int. Ed. 2023, 62, e202304574
DOI: 10.1002/anie.202304574
Angew. Chem. 2023, 135, e202304574
DOI: 10.1002/ange.202304574
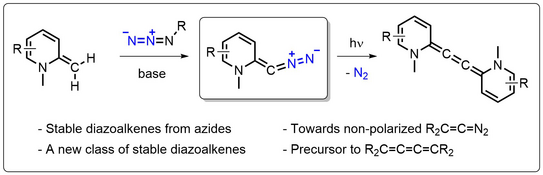
51. J. Reitz, P. W. Antoni, J. J. Holstein, M. M. Hansmann*
Room-Temperature-Stable Diazoalkenes by Diazo Transfer from Azides: Pyridine-Derived Diazoalkenes
Angew. Chem. Int. Ed. 2023, 62, e202301486
DOI: 10.1002/anie.202301486
Angew. Chem. 2023, 135, e202301486
DOI: 10.1002/ange.202301486
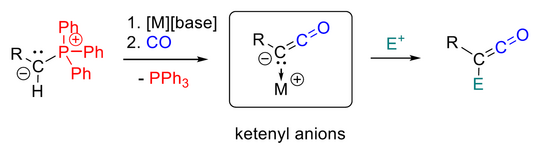
50. M. M. Hansmann*
Transition Metal-Free Activation of Carbon Monoxide: Ketenyl Anions by PPh3/CO Exchange (Highlight)
Angew. Chem. Int. Ed. 2023, 62, e202301826
DOI: 10.1002/anie.202301826
Angew. Chem. 2023, 135, e202301826
DOI: 10.1002/ange.202301826
2022
49. P. W. Antoni, C. Golz, M. M. Hansmann*
Organic Four-Electron Redox Systems Based on Bipyridine and Phenanthroline Carbene Architectures
Angew. Chem. Int. Ed. 2022, 61, e202203064.
DOI: 10.1002/anie.202203064
Angew. Chem. 2022, 134, e202203064
DOI: 10.1002/ange.202203064
2021
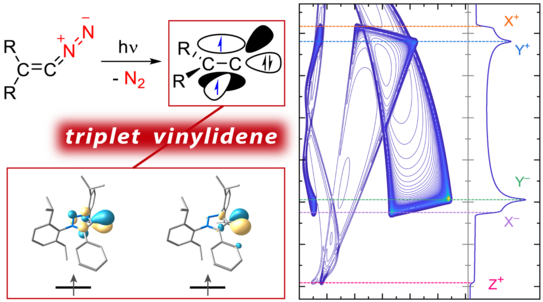
48. Y. Kutin, J. Reitz, P. W. Antoni, A. Savitsky, D. A. Pantazis*, M. Kasanmascheff*, M. M. Hansmann*
Characterization of a Triplet Vinylidene
J. Am. Chem. Soc. 2021, 143, 21410-21415.
DOI: 10.1021/jacs.1c11062
47. P. W. Antoni, J. Reitz, M. M. Hansmann*
N2/CO Exchange at a Vinylidene Carbon Center: Stable Alkylidene Ketenes and Alkylidene Thioketenes from 1,2,3-Triazole Derived Diazoalkenes
J. Am. Chem. Soc. 2021, 143, 12878–12885.
DOI: 10.1021/jacs.1c06906.
Highlighted in "JACS Spotlights" J. Am. Chem. Soc. 2021, 143, 12420–12421.
DOI: 10.1021/jacs.1c08248
46. P. W. Antoni, C. Golz, J. J. Holstein, D. A. Pantazis, M. M. Hansmann*
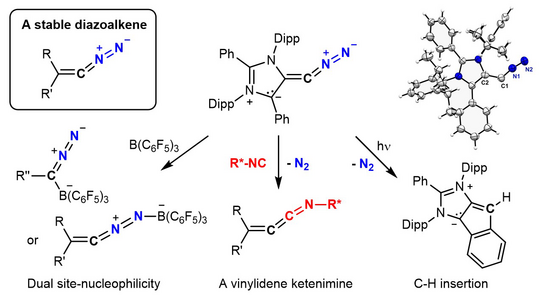
Isolation and reactivity of an elusive diazoalkene
Nat. Chem. 2021, 13, 587–593.
DOI: 10.1038/s41557-021-00675-5.
Highlighted in “Notizen aus der Chemie” Nachr. Chem. 2021, 69 (Juli), 48.
Highlighted in „Trendberichte 2021: Anorganische Chemie“ M. Fischer, D. Heift, Nachr. Chem. 2022, 70 (Feb.), 40-51.
Highlighted in Nat. Chem. 2021, 13, 1030-1032 (DOI: 10.1038/s41557-021-00811-1)
Highlighted in press release of TU Dortmund
Highlighted in the press release of the Federal Ministry of Education and Research (BMBF)
2020
45. M. M. Hansmann*, P. W. Antoni, H. Pesch
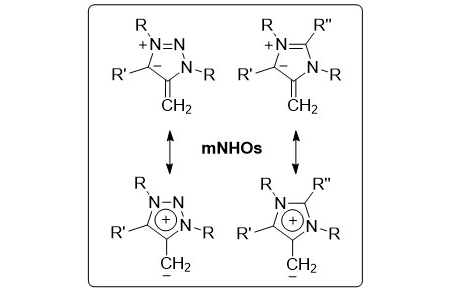
Stable Mesoionic N‐Heterocyclic Olefins (mNHOs)
Angew. Chem. Int. Ed. 2020, 59, 5782-5787.
(DOI: 10.1002/anie.201914571)
Angew. Chem. 2020, 132, 5831-5836.
(DOI: 10.1002/ange.201914571)
Highlighted in „Trendberichte 2020: Anorganische Chemie“ C. Hering-Junghans, C. Sindlinger, Nachr. Chem. 2021, 69, 52-66.
44. J. Messelberger, A. Grünwald, S. J. Goodner, F. Zeilinger, P. Pinter, M. E. Miehlich, F. W. Heinemann, M. M. Hansmann, D. Munz*
Aromaticity and Sterics Control Whether a Cationic Olefin Radical is Resistant to Disproportionation
Chem. Sci. 2020, 11, 4138-4149.
(DOI: 10.1039/D0SC00699H)
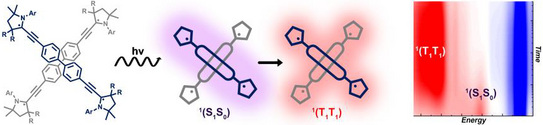
43. T. Ullrich, P. Pinter, J. Messelberger, P. Haines, R. Kaur, M. M. Hansmann*, D. Munz*, D.M. Guldi*
Singlet Fission in Carbene Derived Diradicaloids
Angew. Chem. Int. Ed. 2020, 59, 7906-7914.
(DOI: 10.1002/anie.202001286)
Angew. Chem. 2020, 132, 7980-7988.
(DOI: 10.1002/ange.202001286)
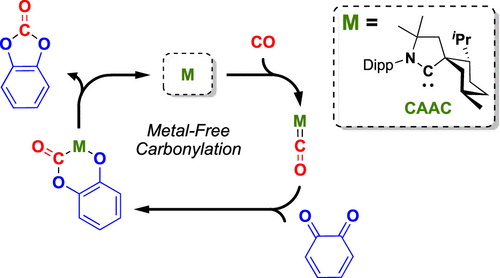
42. J. L. Peltier, E. Tomás-Mendivil, D. R. Tolentino, M. M. Hansmann, R. Jazzar, G. Bertrand*
Realizing Metal-Free Carbene-Catalyzed Carbonylation Reactions with CO
J. Am. Chem. Soc. 2020, 142, 18336-18340.
DOI: 10.1021/jacs.0c09938
2019
41. M. Gosh, H. H. Cramer, S. Dechert, S. Demeshko, M. John, M. M. Hansmann, S. Ye*, F. Meyer*
A μ-Phosphido Diiron Dumbbell in Multiple Oxidation States
Angew. Chem. Int. Ed. 2019, 58, 14349–14356.
(DOI: 10.1002/anie.201908213)
Angew. Chem. 2019, 131, 14487–14494.
(DOI: 10.1002/ange.201908213)
40. P. W. Antoni, T. Bruckhoff, M. M. Hansmann*
Organic Redox-Systems Based on Pyridinium-Carbene Hybrids
J. Am. Chem. Soc. 2019, 141, 9701–9711.
(DOI: 10.1021/jacs.9b04249)
2018
39. P. W. Antoni, M. M. Hansmann*
Pyrylenes: A New Class of Tunable, Redox-Switchable, Photoexcitable Pyrylium-Carbene Hybrids with Three Stable Redox-States
J. Am. Chem. Soc. 2018, 140, 14823–14835.
(DOI: 10.1021/jacs.8b08545)
38. J. Messelberger, A. Grünwald, P. Pinter, M. M. Hansmann, D. Munz*
Carbene derived diradicaloids – building blocks for singlet fission?
Chem. Sci. 2018, 9, 6107-6117.
(DOI: 10.1039/C8SC01999A)
37. M. M. Hansmann*
Synthesis of Azaphosphinines by Directed Inverse Electron Demand Hetero‐Diels‐Alder Reactions with Na(OCP)
Chem. Eur. J. 2018, 24, 11573-11577.
(DOI: 10.1002/chem.201802173)
36. A. Hinz, M. M. Hansmann, G. Bertrand*, J. M. Goicoechea*
Intercepting a Transient Phosphino-Arsinidene
Chem. Eur. J. 2018, 24, 9514-9519. (DOI: 10.1002/chem.201802175)
35. M. M. Hansmann, M. Melaimi, D. Munz, G. Bertrand*
A Modular Approach to Kekulé Diradicaloids Derived from Cyclic (Alkyl)(amino)carbenes
J. Am. Chem. Soc. 2018, 140, 2546-2554. (DOI: 10.1021/jacs.7b11183)
34. M. M. Hansmann, M. Melaimi, G. Bertrand*
Organic Mixed Valence Compounds Derived from Cyclic (Alkyl)(amino)carbenes
J. Am. Chem. Soc. 2018, 140, 2206-2213. (DOI: 10.1021/jacs.7b11184)
2017
33. M. M. Hansmann, M. Melaimi, G. Bertrand*
Crystalline Monomeric Allenyl/Propargyl Radical
J. Am. Chem. Soc. 2017, 139, 15620-15623. (DOI: 10.1021/jacs.7b09622)
32. E. Tomás-Mendivil, M. M. Hansmann, C. M. Weinstein, R. Jazzar, M. Melaimi, G. Bertrand*
Bicyclic (Alkyl)(amino)carbenes (BICAACs): Stable Carbenes more Ambiphilic than CAACs
J. Am. Chem. Soc. 2017, 139, 7753-7756. (DOI: 10.1021/jacs.7b04640)
31. M. M. Hansmann, D. A. Ruiz, L. Liu, R. Jazzar, G. Bertrand*
(Phosphanyl)phosphaketenes as Building Blocks for Novel Phosphorus Heterocycles
Chem. Sci. 2017, 8, 3720-3725. (DOI: 10.1039/C7SC00300E)
30. S. Arndt, M. M. Hansmann, M. Rudolph, F. Rominger, A. S. K. Hashmi*
Direct Access to π-Extended Phosphindolium Salts by Simple Proton-Induced Cyclization of (o-Alkynylphenyl)-phosphanes
Chem. Eur. J. 2017, 23, 5429-5433. DOI: 10.1002/chem.201700889)
29. S. Tšupova, M. M. Hansmann, M. Rudolph, F. Rominger, A. S. K. Hashmi*
Gold-Catalysed Formal Cyclisation/Dimerization of Thiophene-Tethered Diynes
Chem. Eur. J. 2017, 23, 5716-5721. (DOI: 10.1002/chem.201700061)
28. S. Arndt, M. M. Hansmann*, P. Motloch, M. Rudolph, F. Rominger, A. S. K. Hashmi*
Intramolecular anti-Phosphinoauration of Alkynes: An FLP-Motivated Approach to Stable Aurated Phosphindolium Complexes
Chem. Eur. J. 2017, 23, 2542-2547. (DOI: 10.1002/chem.201605914)
2016
27. M. M. Hansmann, G. Bertrand*
Transition-Metal-like Behavior of Main Group Elements: Ligand Exchange at a Phosphinidene
J. Am. Chem. Soc. 2016, 138, 15885-15888. (DOI: 10.1021/jacs.6b11496)
26. G. Kleinhans, M. M. Hansmann, G. Guisado-Barrios, D. C. Liles, G. Bertrand, D. I. Bezuidenhout*
Nucleophilic T-shaped (LXL)Au(I)-pincer complexes: Protonation and Alkylation
J. Am. Chem. Soc. 2016, 138, 15873-15876. (DOI: 10.1021/jacs.6b11359)
Highlighted in ACS Spotlight: J. Am. Chem. Soc. 2016, 138, 16567–16567.
25. S. Tšupova, M. M. Hansmann, M. Rudolph, F. Rominger, A. S. K. Hashmi*
New pathways for the Dual Gold Catalyzed Cyclization of Diynes
Chem. Eur. J. 2016, 22, 16286-16291. (DOI: 10.1002/chem.201602873)
24. L. C. Wilkins, J. R. Lawson, P. Wieneke, F. Rominger, A. S. K. Hashmi, M. M. Hansmann, R. L. Melen*
The Propargyl Rearrangement to Functionalised Allyl- Boron and Borocation Compounds
Chem. Eur. J. 2016, 22, 14618–14624. (DOI: 10.1002/chem.201602719)
23. M. M. Hansmann, R. Jazzar, G. Bertrand*
Singlet (Phosphino)phosphinidenes are Electrophilic
J. Am. Chem. Soc. 2016, 138, 8356-8359. (DOI: 10.1021/jacs.6b04232)
22. M. M. Hansmann, A. López-Andarias, E. Rettenmeier, C. Egler-Lucas, F. Rominger, A. S. K. Hashmi,* C. Romero-Nieto*
B(C6F5)3: A Lewis acid that brings the light to the solid state
Angew. Chem. Int. Ed. 2016, 55, 1196-1199. (DOI: 10.1002/anie.201508461)
Erzeugung von Festkörperlumineszenz durch Koordination der Lewis-Säure B(C6F5)3 an nicht-emittierende Aldehyde
Angew. Chem. 2016, 128, 1212-1216. (DOI: 10.1002/ange.201508461)
(selected as hot paper); Highlighted in “Cutting-Edge Chemistry“ by the ACS.
21. L. C. Wilkins, H. B. Hamilton, B. M. Kariuki, A. S. K. Hashmi, M. M. Hansmann, R. L. Melen*
Lewis acid-base 1,2-addition reactions: Synthesis of pyrylium borates from en-ynoate precursors.
Dalton Trans. 2016, 45, 5929-5932. (DOI: 10.1039/C5DT03340C
2015
20. M. M. Hansmann*, R. L. Melen, M. Rudolph, F. Rominger, H. Wadepohl, D. W. Stephan*, A. S. K. Hashmi*
Cyclopropanation / Carboboration Reactions of Enynes with B(C6F5)3
J. Am. Chem. Soc. 2015, 137, 15469-15477. (DOI: 10.1021/jacs.5b09311)
19. L. C. Wilkins, P. Wieneke, P. D. Newman, B. M. Kariuki, F. Rominger, A. S. K. Hashmi, M. M. Hansmann*, R. L. Melen*
Pathways to functionalized heterocycles: The propargyl rearrangement using B(C6F5)3
Organometallics 2015, 34, 5298-5309. (DOI: 10.1021/acs.organomet.5b00753)
18. E. Rettenmeier, M. M. Hansmann, A. Ahrens, K. Rübenacker, T. Saboo, J. Massholder, C. Meier, M. Rudolph, F. Rominger, A. S. K. Hashmi*
Insights into the Gold-Catalyzed Propargyl Ester Rearrangement/ Tandem Cyclization Sequence: Radical versus Gold Catalysis — Myers–Saito- versus Schmittel-Type Cyclization
Chem. Eur. J. 2015, 21, 14401-14409. (DOI: 10.1002/chem.201501725)
17. R. L. Melen,* L. C. Wilkins, B. M. Kariuki, H. Wadepohl, L. H. Gade, A. S. K. Hashmi, D. W. Stephan, M. M. Hansmann*
Diverging Pathways in the Activation of Allenes with Lewis Acids and Bases: Addition, 1,2-Carboboration, and Cyclization
Organometallics 2015, 34, 4127-4137. (DOI: 10.1021/acs.organomet.5b00546)
2014
16. M. M. Hansmann*, F. Rominger, M. P. Boone, D. W. Stephan*, A. S. K. Hashmi*
Reactivity of Organogold Compounds with B(C6F5)3 – A Gold Boron Transmetalation via σ-B/π-Au Species
Organometallics 2014, 33, 4461-4470. (DOI: 10.1021/om5006885)
15. M. M. Hansmann, R. L. Melen, F. Rominger, A. S. K. Hashmi*, D. W. Stephan*
B(C6F5)3 Promoted Cyclisation of Internal Propargyl Esters: Structural Characterisation of 1,3-Dioxolium Compounds
Chem. Commun. 2014, 50, 7243-7245. (DOI: 10.1039/C4CC01370K) (selected as hot article)
14. T. Wang, S. Shi, M. M. Hansmann, E. Rettenmeier, M. Rudolph, A. S. K. Hashmi*
Synthesis of Highly Substituted 3-Formyl Furans via Gold(I)-Catalyzed Oxidation / 1,2-Alkynyl Migration / Cyclization Cascade
Angew. Chem. Int. Ed. 2014, 53, 3715-3719. (DOI: 10.1002/anie.201310146)
Synthese hochsubstituierter 3-Formylfurane über eine Kaskade aus Gold(I)-katalysierter Oxidation, 1,2-Alkinylwanderung und Cyclisierung
Angew. Chem. 2014, 126, 3789-3793. (DOI: 10.1002/ange.201310146)
Classified as highly cited paper by Elsevier
13. M. M. Hansmann, R. L. Melen, F. Rominger, A. S. K. Hashmi*, D. W. Stephan*
Activation of Alkynes with B(C6F5)3 - Boron Allylation Reagents Derived from Propargyl Esters
J. Am. Chem. Soc. 2014, 136, 777-782. (DOI: 10.1021/ja4110842)
12. M. M. Hansmann*, S. Tsupova, M. Rudolph, F. Rominger, A. S. K. Hashmi*
Gold-catalysed Cyclisation of Diynes: Controlling the Mode of 5-endo vs. 6-endo Cyclisation – An Experimental and Theoretical Case Study Utilizing Diethynylthiophenes
Chem. Eur. J. 2014, 20, 2215-2223. (DOI: 10.1002/chem.201302967)
2013
11. M. M. Hansmann, M. Pernpointner*, R. Döpp, A. S. K. Hashmi*
A Theoretical DFT-based and an Experimental Study of the Transmetalation Step in Au/Pd Mediated Cross-Coupling Reactions
Chem. Eur. J. 2013, 19, 15290-15303. (DOI: 10.1002/chem.201301840)
10. W. Yang, Y. Yu, T. Zhang, M. M. Hansmann, D. Pflästerer, A. S. K. Hashmi*
Gold-Catalyzed Highly Diastereoselective Synthesis of Functionalized 3,4-Disubstituted Butyrolactams via Phosphatyloxy or Carbonate Double Migrations
Adv. Synth. Catal. 2013, 335, 2037-2043. (DOI: 10.1002/adsc.201300158)
Highlighted in SynFacts: Synfacts 2013, 9, 1086.
9. R. L. Melen†, M. M. Hansmann†, A. J. Lough, A. S. K. Hashmi, D. W. Stephan*
Cyclisation versus 1,1-Carboboration: Reactions of B(C6F5)3 with Propargylamides
Chem. Eur. J. 2013, 19, 11928-11938. (DOI: 10.1002/chem.201301899) († equal contribution)
8. M. M. Hansmann, A. S. K. Hashmi*, M. Lautens*
Gold meets Rhodium: Tandem One-Pot Synthesis of β-Disubstituted Ketones via Meyer-Schuster Rearrangement and Asymmetric 1,4-Addition
Org. Lett. 2013, 15, 3226-3229. (DOI: 10.1021/ol4011739)
Highlighted in SynFacts: Synfacts 2013, 9, 1087.
Highlighted on www.organic-chemistry.org
7. M. M. Hansmann*, M. Rudolph, F. Rominger, A. S. K. Hashmi*
Mechanistic switch in dual gold catalysis of diynes: C(sp3)-H activation through bifurcation – vinylidene versus carbene pathways
Angew. Chem. Int. Ed. 2013, 52, 2593-2598. (DOI: 10.1002/anie.201208777)
Mechanistisches Umschalten bei der dualen Goldkatalyse von Diinen: C(sp3)-H-Aktivierung über Bifurkation – Vinyliden- versus Carbenreaktionswege
Angew. Chem. 2013, 125, 2653-2659. (DOI: 10.1002/ange.201208777)
Classified as highly cited paper by Elsevier (> 100 citations)
6. M. M. Hansmann*, F. Rominger, A. S. K. Hashmi*
Gold-Allenylidenes – An experimental and theoretical study
Chem. Sci. 2013, 4, 1552-1559. (DOI: 10.1039/C3SC22227F)
5. A. S. K. Hashmi*, W. Yang, Y. Yu, M. M. Hansmann, M. Rudolph, F. Rominger
Gold-catalyzed formal 1,6-acyloxy migration leading to 3,4-disubstituted pyrrolidin-2-ones
Angew. Chem. Int. Ed. 2013, 52, 1329-1332. (DOI: 10.1002/anie.201207287)
Goldkatalysierte formale 1,6-Acyloxywanderung unter Bildung von 3,4-disubstituierten Pyrrolidin-2-onen
Angew. Chem. 2013, 125, 1368-1371. (DOI: 10.1002/ange.201207287)
Highlighted in SynFacts: Synfacts 2013, 9, 414.
2012
4. B. M. Trost*, D. A. Thaisrivongs, M. M. Hansmann
Tandem Palladium(0)- and Palladium(II)-catalyzed allylic alkylation through complementary redox cycles
Angew. Chem. Int. Ed. 2012, 51, 11522-11526. (DOI: 10.1002/anie.201204251)
Angew. Chem. 2012, 124, 11690-11694. (DOI: 10.1002/ange.201204251)
3. B. M. Trost*, M. M. Hansmann, D. A. Thaisrivongs
Palladium-catalyzed alkylation of 1,4-dienes by C–H activation
Angew. Chem. Int. Ed. 2012, 51, 4950-4953. (DOI: 10.1002/anie.201200601)
Angew. Chem. 2012, 124, 5034-5037. (DOI: 10.1002/ange.201200601)
2011
2. A. S. K. Hashmi*, M. Pernpointner, M. M. Hansmann
Theoretical insights into the superior activity of gold catalysts and reactions of organogold intermediates with electrophiles
Faraday Discuss. 2011, 152, 179-184. (DOI: 10.1039/C1FD00029B)
1. M. M. Hansmann, R. L. Melen, D. S. Wright*
Group 13 BN dehydrocoupling reagents, similar to transition metal catalysts but with unique reactivity
Chem. Sci. 2011, 2, 1554-1559. (DOI: 10.1039/C1SC00154J)
Patents
1. Novel compounds exhibiting photopysical properties upon formation of Lewis acid-base adducts using non-chelating boranes, method for producing the same and devices including the same.
M. M. Hansmann, A. S. K. Hashmi, C. Romero-Nieto, A. López-Andarias, E. Rettenmeier, C. Egler-Lucas
Patent registration WO 2017/016653 A1; PCT/EP2016/001254.
Non-Peer Reviewed Articles
M. M. Hansmann*
Blickpunkt Nachwuchs: Redoxsysteme und neue stabile Verbindungsklassen
Nachrichten aus der Chemie, 2022, 70, 80-81
DOI: 10.1002/nadc.20224125418