Part of the turbo-Grignard puzzle unraveled
The field of application of organo-magnesium halides, named as Grignard reagents after Victor Grignard (Nobel laureate in 1912), covers many areas of synthetic chemistry such as simple electrophilic addition and halogen exchange reactions, precursors of complex coupling reactions, and the synthesis of optoelectronically active polymers. These commonly used and commercially available reagents are still the subject of intensive and extensive scientific research more than 100 years after their discovery. The reason for this, besides the continuous development of the technologies and the reagents themselves, is the high number of equilibria that are formed between the possible structures. These equilibria are summarized by the Schlenk equilibrium and have since been regarded as a "chemical puzzle" in the context of organo-metallic chemistry.
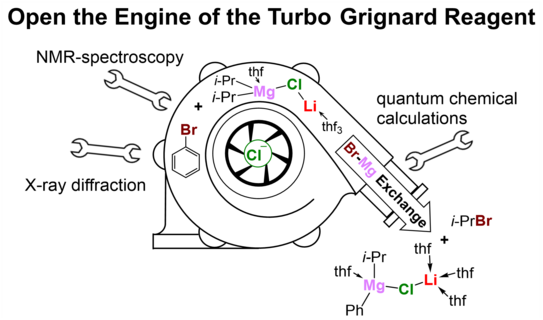
The development of the Turbo-Grignard reagent by Paul Knochel took the organo-magnesium halide reagent class to a whole new level. In this process, a significant increase in reactivity and chemoselectivity was achieved by the simple addition of lithium chloride. While an increase in yield and a reduction in reaction time were observed, the reaction conditions applied were milder and the tolerance to functional groups was higher. However, many studies aimed at elucidating a possible reactive species have so far only been able to provide insight into the high diversity and high dynamic behavior of the Schlenk equilibrium.
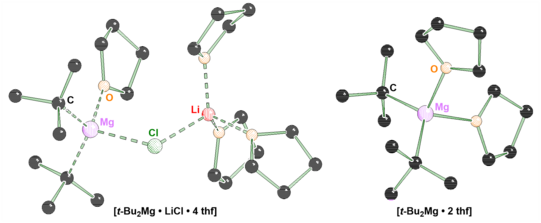
A cooperation group of the TU Dortmund University consisting of members of the working group of Prof. Dr. Carsten Strohmann and the NMR team of Prof. Dr. Wolf Hiller has now succeeded in identifying a possible reactive species of the Turbo-Grignard reagent in the solid state, detecting it in solution and verifying it by means of quantum chemical calculations. This deeper study provides a possible explanation for the frequently observed reactivity enhancement of the Turbo Grignard reagent, in that the incorporation of the lithium chloride into a diorgano-magnesium compound can energetically stabilize the transition state of corresponding reactions. The formation of this bimetallic species from a stable diorgano-magnesium compound and a formal salt seems surprising at first, but is shown to be part of deciphering the Turbo-Grignard puzzle. The paper was published in the prestigious journal Angewandte Chemie.
Comprehensive Study of the Enhanced Reactivity of Turbo-Gignard-Reagents
A. Hermann, R. Seymen, L. Brieger, J. Kleinheider, B. Grabe, W. Hiller, C. Strohmann*
Angew. Chem. Int. Ed. 2023, e202302489.