New Insights into the Fractional Crystallization of Si-Stereogenic Silanes for Stereospecific Processes
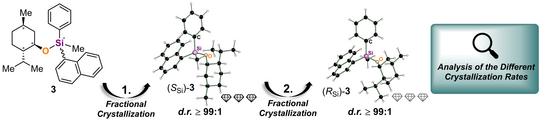
In chemistry, silicon-stereogenic compounds play a significant role, particularly in stereospecific processes. This new work builds on a method by Sommer et al., which produces diastereomeric silicon compounds through fractional crystallization. However, previous studies focused on the crystallization of one of the (SSi)- and (RSi)-diastereomers, with the (SSi)-diastereomer crystallizing faster. A selective fractional crystallization of both diastereomers would represent an atom-economic synthetic access for both chiral menthoxysilanes.
The approach presented by Sommer et al. in the 1960s was thoroughly analyzed to gain insights for efficiently separating both diastereomers with excellent stereochemical purity. During these studies, both diastereomers, (SSi)-3 und (RSi)-3, were isolated in good yields and fully characterized. By employing single-crystal X-ray diffraction analyses of both solid structures, followed by Hirshfeld analysis, differences in crystal structure were identified that could explain the varying crystallization rates. Additionally, DFT calculations indicate that both diastereomers show similar ground state energies. Hence, the selective approach may be attributed to interactions within the solid-state structures. Initial NMR studies confirmed the configurational stability of the menthoxysilanes under elevated reaction temperatures and basic pH levels. Therefore, they represent particularly interesting starting materials for stereospecific transformations, which can be performed with both isolated compounds.
Supramolecular Interactions as Key in Racemic Resolution: New Insights into the (SSi)- and (RSi)-Diastereomers of (1R,2S,5R)-Methyl(1-naphthyl)-phenylmenthoxysilane of Sommer
J.-L. Kirchhoff, C. Strohmann,
Z. anorg. allg. Chem. 2024, 650, e202400135.