New Insights in Halogen Bonds to Simple Ethers
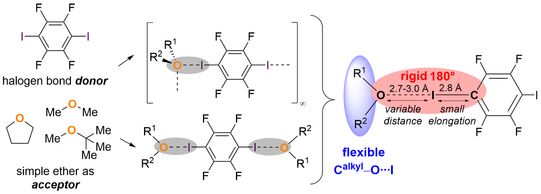
Our publication deals with the so far less researched topic of halogen bonds to dialkyl ethers. The focus is on the synthesis and structural analysis of the first halogen bonds with non-cyclic alkyl ethers with the halogen bond donor 1,4-diiodotetrafluorobenzene. Three common ethers - dimethyl ether, tetrahydrofuran and methyl tert-butyl ether - were used as acceptors. Two different structural motifs were obtained: Discrete trimolecular aggregates on the one hand, and supramolecular chain adducts characterized by oxygen-bifurcated halogen bonds on the other. Depending on the stoichiometry used, both structures can also be selectively generated with the same ether. The obtained highly sensitive structures emphasize the versatility of ethers as halogen bond acceptors, which can occur in different geometrical arrangements and thus could play an important role in many structural designs in nature.
In the following, the observed flexibility of the geometric arrangement around the oxygen center of the ethers, in contrast to the almost linear arrangement around the halogen atom, is considered. High-resolution X-ray diffraction experiments on the dimethyl ether adduct allowed a detailed investigation of the electron density at the bond sites. The data indicate that the halogen bond is predominantly electrostatic in nature, which also explains the observed geometric flexibility of the ethers.
The Halogen Bond to Ethers - Prototypic Molecules and Experimental Electron Density
A. Schmidt, A. Krupp, J. Kleinheider, T. M. L. Binnenbrinkmann, R. Wang, U. Englert*, C. Strohmann*
ACS Omega, 2024, 9, 35037–35045.





