Novel "Frustrated" Aggregates: Enhancing t-BuLi Reactivity with THF in n-pentane
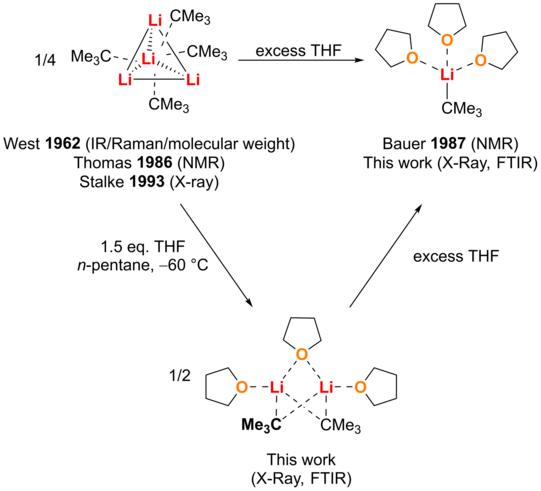
In alkyllithium chemistry, the highest reactivity is traditionally associated with the lowest degree of aggregation, so tert-butyllithium in tetrahydrofuran solution is considered a reactive system. The work presented here presents a significant increase of the reactivity of t-BuLi by using stoichiometric amounts of THF in n-pentane to enable the deprotonation of simple methylsilanes and other substrates with low C-H acidity. The obtained aggregates of t-BuLi with THF were investigated by single-crystal X-ray structural analysis, in situ FTIR spectroscopy and quantum chemical calculations. Surprisingly, the structural analysis revealed a bridged dimer, which was different from the previously known monomers and dimers. Experimental results, especially the in situ FTIR studies, support the existence of this bridged dimer and suggest that they are "frustrated" aggregates characterized by the weak coordination of the THF ligand.
The use of stoichiometric amounts of THF in n-pentane led to a significant increase in the reactivity of t-BuLi, especially in the deprotonation of tetramethylsilane. Quantum chemical calculations confirm these observations. The study concludes by presenting these novel "frustrated" aggregates as a target for the deaggregation of alkyl lithium reagents and emphasizes the importance of targeted studies of solvent mixtures for optimized reaction conditions. The paper was published in Chemistry A European Journal.