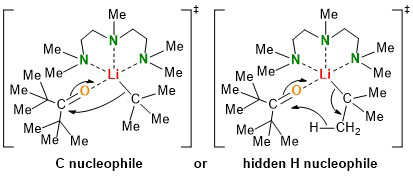
Lithium alkyls as source for lithium hydride?
Lithium alkyls have a wide range of applications. Due to the polar carbon-lithium bond, they are suitable both as a Brønsted base and as a carbon nucleophile. Fully understanding the selectivity of the reactions that take place is still part of current research in our own working group. In this field, the new publication is a further step towards understanding lithium alkyl chemistry. By using sterically demanding ligands and the sterically demanding hexamethylacetone, an addition of lithium hydride was observed instead of the expected nucleophilic addition of the lithium alkyls used.
Experiments showed an increased tendency of β-hydride elimination and lithium hydride addition with increasing steric demand of the ligand and the lithium alkyl used. However, it should be noted that the lithium alkyls used should be stable against decomposition at the very low temperatures. In the case of sec-butyllithium, it was possible to change the selectivity from a previously exclusive addition reaction without ligand to a selective lithium hydride transfer upon addition of the ligand PMDTA. No lithium hydride transfer was observed when primary lithium alkyls were used. Subsequent quantum chemical calculations confirmed the experimental trends.
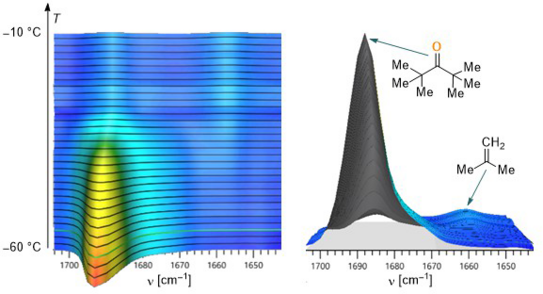
In addition, the hypothesis of simultaneous β-hydride elimination and lithium hydride addition was developed for the mechanism of lithium hydride transfer, which was confirmed by subsequent in situ FTIR spectroscopic investigations. The publication was published in the well-known journal Organometallics.
Alkyllithium Reagents as a Hidden Source of Lithium Hydride
J. Kleinheider, C. Schwab, C. Strohmann*
Organometallics 2023, 42, 3173–3177.