Configuration Studies of Palladium Complexes
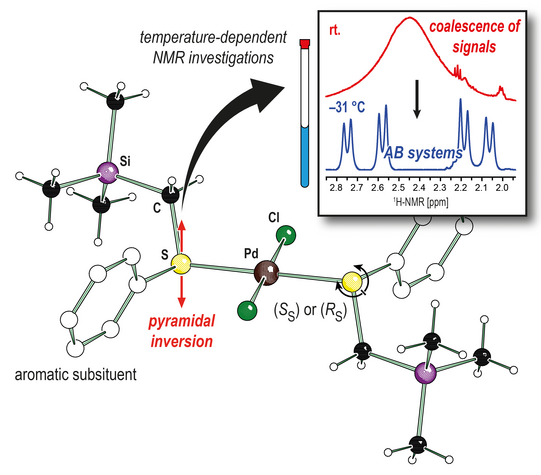
Thioethers are a frequently used ligand class in the coordination chemistry of transition metals. For example, their palladium(II) complexes are known to promote hydrolysis of oligopeptides with outstanding regioselectivity or to co-polymerize carbon monoxide and tert-butylstyrene. The properties of the complexes can be significantly influenced and thus controlled by the substitution pattern of the ligand. One of these properties is the inversion stability of the coordinating sulphur atoms. By forming a coordinative bond with one of the lone pairs, the sulphur atom in asymmetrically substituted thioethers becomes stereogenic center, which can invert by pyramidal inversion.
To investigate the configurational stability of square-planar palladium(II) chloride complexes in relation to the substitution of the thioether ligand, we synthesized a series of three trimethylsilyl-substituted thioanisole derivatives and their corresponding complexes. Single-crystal X-ray structure analysis gave unlike configurated complexes for the two less sterically demanding thioether ligands, and like diastereomers with the most sterically demanding ligand in the solid-state. Temperature-dependent NMR studies showed that at room temperature inversion processes at the sulfur atoms occur with rates comparable to the 1H NMR time scale. The signals coalescing at 24 °C split into double data sets when the measuring temperature was lowered, caused by the presence of both diastereomers.
Synthesis of Organosilicon-Based Thioether Ligand Class Showing Defined S-Stereogenic Coordination in Crystalline Palladium(II) Complexes
P. E. Schneider, J. Wattenberg, J. F. Wappelhorst, M. Knorr, C. Strohmann*
Z. Allg. Anorg. Chem. 2023, 649, e202300185.