Insights into the Molecular Halogen Bond
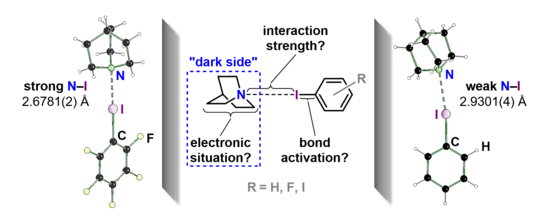
Halogen bonds (XBs) are interactions between a heavy halogen atom (XB donor) and a nucleophile (XB acceptor) such as nitrogen, oxygen or other halides. In this process, electron-withdrawing substituents polarize the electron shell of the XB donor in such a way that a positive potential is created in the opposite direction to the substituent, which is called a σ-hole. Due to this strong linearity, which results structurally from the donor electron pair-σ-hole interaction, many applications of halogen bonds can be found.
Among these, halogen bonds are used in organocatalysis, crystal engineering, and drug discovery. Often, a combination of several halogen bonds and also other interactions such as hydrogen bonds or London dispersion forces play an important role in these fields of application. Thus, investigating an isolated halogen bond for the exact effects that occur, especially at the XB acceptor, shows promise for better assessing the influence of the halogen bridge in the aforementioned fields of application.
This question was addressed by a consortium of chemists from the TU Dortmund University, the Ruhr University Bochum and the RWTH Aachen. The molecular halogen bonds of iodobenzene and 1,4-diiodobenzene and quinuclidine were compared with the respective perfluorinated XB donors. In an extensive experimental study consisting of experimental electron density studies, 1D-NMR titrations and RAMAN spectroscopy, a correlation from the strength of the halogen bond and the change in the electronic situation at the electron donor was experimentally demonstrated for the first time. The manuscript has been published in the emerging open access journal ACS Omega.
Gauging the Strength of the Molecular Halogen Bond via Experimental Electron Density and Spectroscopy
F. Otte, J. Kleinheider, B. Grabe, W. Hiller, F. Busse, R. Wang, N. M. Kreienborg, C. Merten*, U. Englert*, C. Strohmann*
ACS Omega 2023, accepted.