Research
Outline
Metal-organic frameworks (MOFs) represent a diverse and highly tuneable family of inorganic-organic hybrid materials exhibiting large specific surface areas and huge pore volumes. The zeolite-like architectures are composed of metal ions, which are interconnected by organic linker molecules (multivalent carboxylates, imidazolates, etc.) to generate two- or three-dimensional extended networks.
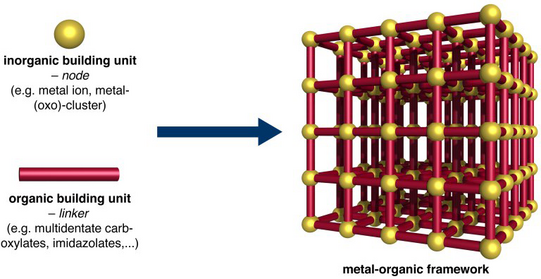
Based on a large variety of molecular building units an almost infinite number of MOFs could in principle be synthesised. Simple exchange of the inorganic and/or the organic units allows for a targeted tuning of the framework properties by means of synthetic chemistry. In view of their huge potential for various applications such as hydrogen storage, carbon dioxide capture, catalysis or drug delivery, MOFs attract tremendous interest from academia as well as industry.
Rational synthesis of functionalised metal-organic frameworks
Via rational design of the organic linkers we aim to design the structure as well as the functional properties of MOFs. Currently we are developing synthetic strategies for alkali ion based MOFs. MOFs based on group 2, d-block, group 13 or lanthanide ions are abundant, while alkali-MOFs are extremely rare. Such materials, however, are interesting for electrochemical energy storage or as solid electrolytes and further open up new possibilities because they can be processed from aqueous solution.
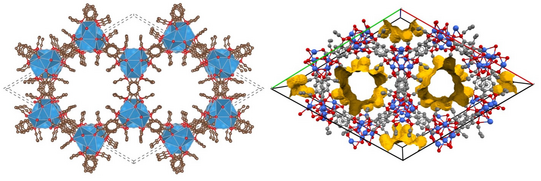
Understanding responsive metal-organic frameworks
We study the responsive behaviour of MOFs with respect to guest inclusion, changes in temperature or application of mechanical pressure with state-of-the-art X-ray diffraction (XRD) and gas/vapour sorption techniques. Our XRD experiments are often done in situ with highly intense synchrotron radiation either at the Dortmund synchrotron DELTA or at larger facilities such as DESY (Hamburg) or Diamond Light Source (near Oxford, UK).
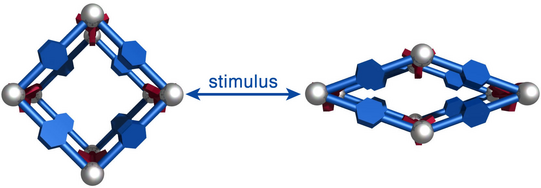
Just recently, we discovered that specific MOFs based on tetrahedrally coordinated divalent metal ions (Zn2+ or Co2+) and imidazolate linkers switch from an open to a closed form when compressed hydrostatically. In this process, no chemical bond is broken while the crystals reduce their volume by more than 20%. Once the pressure is released, the MOFs return to the open pore form. Such transitions may pave the way for applications of MOFs as shock absorbers, nanodampers or for solid-state refrigeration applications.
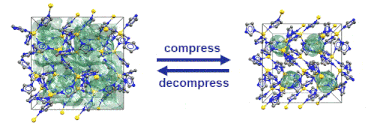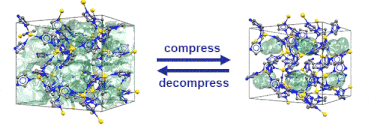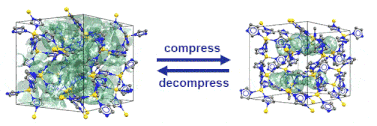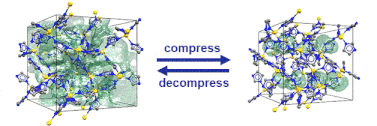
”Pore closure in zeolitic imidazolate frameworks under mechanical pressure”
S. Henke et al. Chem. Sci. 2018, 9, 1654-1660.
Porous metal-organic framework liquids and glasses
MOF glasses represent a very new class of functional materials which can be obtained from meltable MOFs by vitrification of the melt. MOFs liquids and glasses have a major advantage against their crystalline counterparts, as they can be processed and shaped into different forms before vitrification to porous MOF glasses. Surprisingly, there are only little more than a handful of MOFs which can be melted and transformed into glasses. Thousands of other MOFs simply decompose upon heating without melting.
We want to understand the chemical and structural features, which are required to melt MOFs. We further look into the textural properties (porosity, pore size, surface area, etc.) of MOF glasses, which we want to tune by building block design. Recently, we prepared the very first cobalt-based zeolitic imidazolate framework (ZIF) that can be melted and transformed into a nanoporous glass. These findings might pave the way for the application of liquid and glassy MOFs in gas separation processes and catalysis.
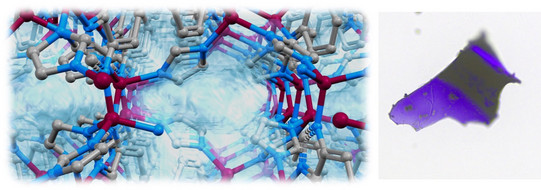
”Porous purple glass - A cobalt imidazolate glass with accessible porosity from a meltable cobalt imidazolate framework”
L. Frentzel-Beyme et al. J. Mater. Chem. A 2019, 7, 985-990.
"Meltable Mixed-Linker Zeolitic Imidazolate Frameworks and Their Microporous Glasses - From Melting Point Engineering to Selective Hydrocarbon Sorption”
L. Frenzel-Beyme et al. J. Am. Chem. Soc. 2019, 141, 12362-12371.





