Research
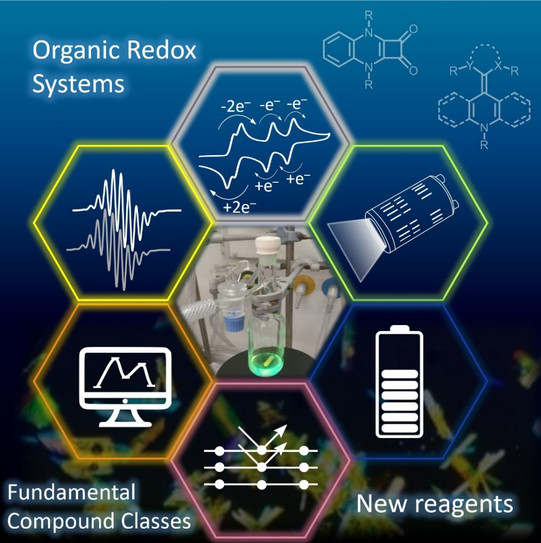
The Hansmann group is a synthetic organic chemistry group with overlap into the areas of main-group chemistry, reactive intermediates, spectroscopy, electro chemistry, supported by computational studies. Current research includes the following research areas I-III:
Area I: Fragment Transfer Reagents for Organic Synthesis
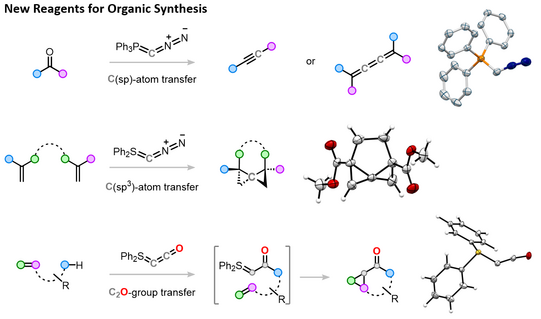
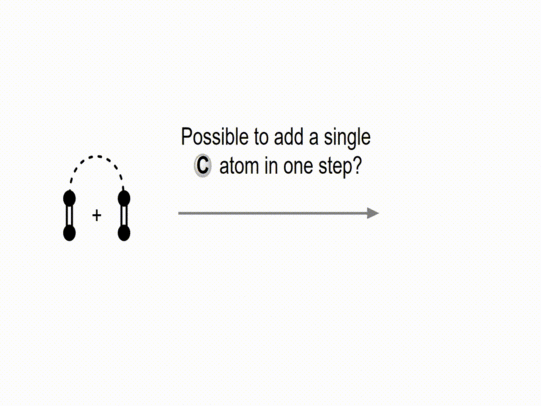
The direct transfer of carbon atoms to organic substrates, enabling the formation of new carbon–carbon bonds, constitutes a synthetically highly valuable transformation as it permits the direct and efficient construction and modification of carbon frameworks. Despite its potential, general and operationally safe precursors for single-carbon incorporation remain scarce. Recently, our group has developed a class of reagents capable of mediating C-atom transfer, based on ylides with the structures R₃PCN₂ and R₂SCN₂ (Figure 1). These reagents enable the direct introduction of C(sp) and C(sp³)-atoms to afford alkynes, butatrienes, and even 3D structures such as (bridged) spiropentanes. Moreover, this concept has been extended to reactive fragments such as CN₂ and CCO moieties, providing access to pyrazoles as well as bicyclic scaffolds incorporating cyclopropane rings.
Representative Publications in Area I:
Angew. Chem. Int. Ed. 2025, 64, e202518689.
Science 2025, 387, 885–892.
Science 2024, 385, 305–311.
Area II: Reactive Molecules – Mesoionic Olefins, Diazoalkenes and Diradicals
Our research focuses on the investigation of reactive intermediates and unconventional classes of organic compounds. We have reported, for the first time, strongly polarized olefins –mesoionic N‑heterocyclic olefins (mNHOs) – derived from triazole, imidazole, and even pyridine frameworks (Figure 2). The nucleophilicity of these species has been quantified, revealing exceptionally high N values on the Mayr nucleophilicity scale. In addition to acting as potent carbon‑centered nucleophiles, mNHOs can also behave as 1,3‑dipoles and have found applications in catalysis for instance CO2 functionalization chemistry.
Furthermore, our group discovered the first room-temperature stable diazoalkenes. While diazoalkenes (R2C=C=N2) had previously been considered as elusive intermediates, electronic stabilization enabled the isolation of a series of stable heterocyclic based diazoalkenes. These compounds exhibit ligand‑exchange reactivity in which the diazo moiety can be replaced by small molecules such as CO, isocyanides, or carbenes, resulting in novel exchange processes. Moreover, the CN₂ fragment itself can engage in 1,3‑dipolar reactions – for example, activation of white phosphorus leads to the formation of diazadiphospholes.
Photoinduced release of dinitrogen from cumulenic diazo compounds generates novel classes of carbon‑centered diradicals. We have characterized the first triplet vinylidenes as well as ligand‑stabilized (PPh₃) carbon diradicals. Typically, these species are generated at low temperatures in an organic glass and studied using electron paramagnetic resonance (EPR) spectroscopy.
Representative Publications in Area II:
Angew. Chem. Int. Ed. 2026, 65, e16032.
JACS Au 2025, 5, 2884–2897.
Angew. Chem. Int. Ed. 2025, 64, e202506305.
Angew. Chem. Int. Ed. 2025, 64, e202424166.
Angew. Chem. Int. Ed. 2025, 64, e202415228.
Angew. Chem. Int. Ed. 2024, 63, e202318283.
Angew. Chem. Int. Ed. 2023, 62, e202309790.
Angew. Chem. Int. Ed. 2023, 62, e202301486.
J. Am. Chem. Soc. 2021, 143, 12878–12885.
Nat. Chem. 2021, 13, 587–593.
Angew. Chem. Int. Ed. 2020, 59, 5782-5787.
Area III: Organic Redox Systems – Photocatalyst Design and Energy Storage
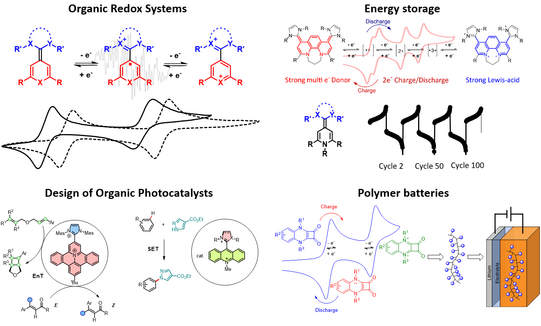
Our group has developed a series of novel redox systems based on the combination of stable carbenes with cationic heterocycles such as pyrylium, pyridinium, and acridinium salts (Figure 3). The resulting neutral hybrids typically exhibit three-stage redox behavior, with each oxidation state being accessible and isolable as a stable compound. In addition, we are pursuing the design of multi-electron redox systems capable of reversibly storing more than two electrons. Such systems are investigated in energy storage technologies, including redox-flow and polymer-based batteries. Upon photoexcitation, these carbene–heterocycle assemblies can also function as new classes of organic photocatalysts. We have employed them in challenging oxidative photocatalytic transformations as well as in energy-transfer catalysis.
Representative Publications in Area III:
ACS Catal. 2025, 15, 16718–16730
J. Am. Chem. Soc. 2024, 146, 3416–3426
J. Am. Chem. Soc. 2023, 145, 23334–23345.
Angew. Chem. Int. Ed. 2022, 61, e202203064.
J. Am. Chem. Soc. 2019, 141, 9701–9711.
J. Am. Chem. Soc. 2018, 140, 14823–14835.