Easy access to chiral phosphanes found
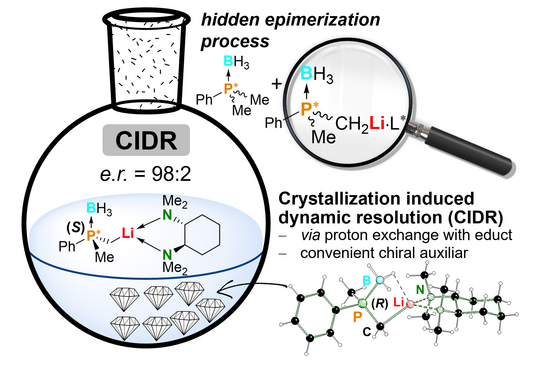
Phosphorus-stereogenic compounds are required for numerous applications in a wide range of fields. They are particularly important as ligands for asymmetric transition metal catalysis (Nobel Prize 2001). Therefore, their targeted and selective synthesis is equally essential. Existing synthesis methods are based on cost-intensive chiral auxiliaries and often limited enantiomeric purities. Through a deep insight into a novel epimerization process in solution, we have now provided a cost-efficent, simple and successful approach to chiral phosphines based on crystallization-induced dynamic resolution.
Crystallization-induced dynamic resolution is based on the favored crystallization of only one diastereomer of an epimeric mixture. This method has now been applied to the deprotonation of an important phosphorus building block. In the presence of the chiral low-cost auxiliary (R,R)-TMCDA, only one lithiated diastereomer crystallized out of the reaction solution. After separation of these crystals, the lithiated compound can subsequently be used to obtain chiral phosphorus compounds with high selectivity.
However, the diastereomer remaining in the solution transforms into the crystallizing species in the presence of non-lithiated reactant and reproduces it in the reaction solution, so that equally high yields were obtained. By NMR-based isotope labelling experiments and complementary quantum chemical calculations, we showed that the phosphorus center of a-lithiated phosphinoboranes, previously thought to be stable, is not stereostable in the presence of excess reactant. This paves the way for many other possible techniques in order to synthesize chiral phosphines and a possible revision of systems already considered to have failed. The publication was published in the prestigious journal Angewandte Chemie as a “Hot Paper”. We thank Fonds der Chemischen Industrie and Avicenna-Studienwerk for the financial support.





